Evaluating Dose-Dependent Skin Changes from Long-Term Isotretinoin Therapy, Including CHE
Key Takeaways
- A five-year study on isotretinoin for acne found skin dryness as a common adverse effect, with chronic hand eczema correlating with higher dosages.
- Common adverse effects included xerosis, retinoid dermatitis, and cheilitis, with lipid profile deviations noted, particularly in cholesterol and LDL levels.
New research highlights the link between isotretinoin dosage and increased chronic hand eczema, emphasizing the need for careful monitoring during acne treatment.
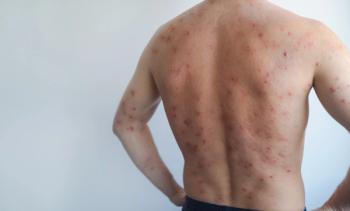
New research evaluates the adverse effects of oral isotretinoin for acne throughout 5 years of treatment.1 The majority of these were skin-related changes, particularly related to the dryness of the skin in various regions, including the hands.
Methods and Materials
The retrospective study involved 370 Polish patients with a mean age of 28 years. All were at least 15 years old at the beginning of therapy. Approximately 71% were female. The participants were treated with isotretinoin for a confirmed diagnosis of acne sometime between June 2020 and June 2025. A control group was also selected and included 300 participants between the ages of 20 and 40 (mean age = 29.44).
The average daily dose was 23.4 mg/day (range: 4.6–50; median 20.0; IQR 20.0–30.0). According to the authors, the optimal dosage of isotretinoin is 0.5 mg/kg of body weight/day for about the first 2 to 4 weeks, then a maintained dosage of 0.5–1 mg/kg of body weight/day for about 4 to 6 months.2
Follow-up visits were held every 2 months to assess physical progress as well as clinical adverse reactions. The documented adverse effects were categorized into these groups: skin changes, oral changes, nasopharyngeal changes, ophthalmic changes, musculoskeletal changes, gastrointestinal changes, infections, mood and neurological changes, and others.
Results
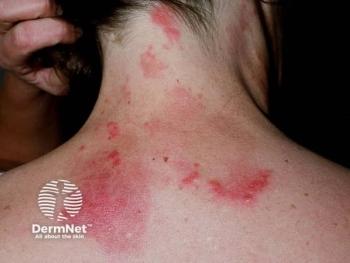
As daily dosages of isotretinoin increased, rates of chronic hand eczema (CHE) also rose, indicating a positive correlation (ρ = 0.082; p = 0.037). This occurred in 3.5% of patients. Prior research supports this, stating that dryness-related skin changes directly depend on the dosage levels.3 Pruritus (8.4%), seborrhea (3.5%), and desquamation (3.25%) were also observed.
Other common adverse events include xerosis (70%), retinoid dermatitis (20%), cheilitis (15.5%), and fatigue (7.8%). Most adverse effects fell into the “skin changes” category. Mood-related adverse events were minimal. Xerosis and cheilitis were some of the earliest symptoms, occurring within the first week of therapy initiation. The peak of dryness was seen in week 4.
Other correlations between adverse effects and clinical variables were found. Retinoid dermatitis occurred more frequently in younger patients, for example (ρ = −0.080, p = 0.029). Conversely, desquamation was more prominent in older participants (ρ = +0.083, p = 0.023).
“Overall, these correlations were statistically significant but weak in magnitude, suggesting that while dose and age may influence the occurrence of certain adverse effects, their clinical impact in isolation is likely limited,” the authors wrote.
There were also deviations in lipid profile that were outside the normal range in the group of patients treated with isotretinoin, particularly in total cholesterol, thyroid-stimulating hormone, and LDL levels. These abnormalities typically became apparent by the fourth week. Furthermore, patients aged 15 to 25 years who received isotretinoin had a statistically significant positive correlation between their daily isotretinoin dose and LDL levels.
Conclusion
Isotretinoin was generally well tolerated at moderate cumulative doses and overall, the frequency of adverse events was similar or even lower in this study compared to previous research. However, rates of CHE, xerosis, and other dry-skin dermatoses, as well as disturbances in lipid and prolactin levels, were seen. To combat this, the authors recommend routine profile monitoring, dose individualization, and the early use of emollients on affected areas.
References
1. Feszak IJ, Brzeziński P, Feszak S, et al. Isotretinoin Treatment for Acne Vulgaris: A Five-Year Retrospective Analysis of Clinical and Biochemical Adverse Effects. J Clin Med. 2025;14(18):6473. Published 2025 Sep 14. doi:10.3390/jcm14186473
2. Akyol, M., & Özçelik, S. (2005). Non-acne dermatologic indications for systemic isotretinoin. American Journal of Clinical Dermatology, 6(3), 175-184.
3. Bagatin, E., & Costa, C. S. (2020). The use of isotretinoin for acne–an update on optimal dosing, surveillance, and adverse effects. Expert Review of Clinical Pharmacology, 13(8), 885-897.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.