AVTX-009 Completes Enrollment in HS Phase 2 Trial
Key Takeaways
- AVTX-009 targets IL-1β to reduce inflammation in hidradenitis suppurativa, potentially offering a new therapeutic approach distinct from TNF inhibitors.
- The LOTUS trial is a randomized, double-blind, placebo-controlled study with primary and secondary endpoints assessing efficacy and patient-reported outcomes.
Avalo Therapeutics completes enrollment in the LOTUS trial for AVTX-009, targeting hidradenitis suppurativa with promising IL-1β inhibition.
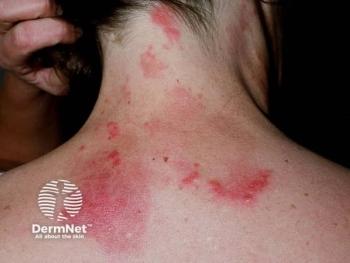
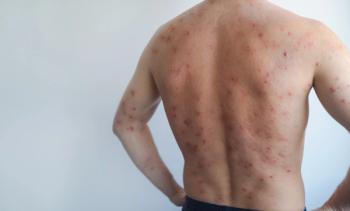
Avalo Therapeutics has announced the completion of patient enrollment in its phase 2 LOTUS trial evaluating AVTX-009 in adults with hidradenitis suppurativa (HS). The trial exceeded its target enrollment of 222 participants, enrolling approximately 250 adults with moderate to severe HS. Topline results from this study are expected in mid-2026.1
Background and Rationale
Hidradenitis suppurativa is often associated with significant pain, scarring, and impaired quality of life. Although tumor necrosis factor (TNF) inhibitors, such as adalimumab, are currently approved for the treatment of HS, therapeutic responses remain suboptimal for many patients, and recurrence after treatment discontinuation is common.2
Emerging evidence has implicated the IL-1 signaling pathway, particularly interleukin-1 beta (IL-1β), in the pathogenesis of HS. Elevated IL-1β expression has been observed in HS lesions and serum, correlating with disease severity. Inhibition of IL-1β may therefore represent a promising therapeutic strategy targeting upstream inflammatory mechanisms that drive neutrophilic infiltration, follicular occlusion, and abscess formation.
AVTX-009, Avalo Therapeutics’ lead clinical candidate, is a high-affinity monoclonal antibody designed to neutralize IL-1β. By inhibiting this proinflammatory cytokine, the agent aims to reduce the chronic inflammatory cascade implicated in HS lesion formation and maintenance.
Study Design and Objectives
The LOTUS trial (
Participants were randomized in a 1:1:1 ratio to receive one of two dose regimens of AVTX-009 or placebo during a 16-week treatment phase. The trial’s primary endpoint is the proportion of participants achieving Hidradenitis Suppurativa Clinical Response 75 (HiSCR75) at Week 16. HiSCR75 is defined as at least a 75% reduction in total abscess and inflammatory nodule count with no increase in abscesses or draining fistulas relative to baseline.
Secondary endpoints include:
- Proportion of participants achieving HiSCR50 and HiSCR90,
- Change from baseline in International HS Severity Score System (IHS4),
- Change in draining fistula count, abscess and inflammatory nodule count,
- Proportion of participants achieving at least a 30% reduction in Patient’s Global Assessment of Skin Pain (PGA Skin Pain).
These endpoints are consistent with current clinical trial standards for HS and aim to capture both objective inflammatory activity and patient-reported pain outcomes.
Clinical and Research Implications
Completion of enrollment represents a key milestone for the AVTX-009 development program. The study’s design, with 2 dosing regimens and robust efficacy measures, may help delineate optimal therapeutic dosing and clarify the degree to which IL-1β inhibition can modify HS disease activity.
While IL-1β has been implicated in multiple autoinflammatory and autoimmune diseases, its role in HS is of particular interest due to the disease’s mixed inflammatory signature involving both innate and adaptive immune pathways. Previous studies of IL-1 blockade (e.g., anakinra) have demonstrated variable results, possibly related to dosing, disease severity, or patient heterogeneity. If AVTX-009 demonstrates significant efficacy and an acceptable safety profile, it may provide a differentiated approach distinct from TNF inhibition, potentially expanding the therapeutic armamentarium for HS management.
Looking Ahead
According to Avalo Therapeutics’ Chief Executive Officer, Garry Neil, MD, the completion of enrollment in the LOTUS trial underscores strong investigator and patient engagement and reflects the ongoing unmet clinical need in HS. The company anticipates topline efficacy and safety data in mid-2026.
Until these data are available, the clinical utility of IL-1β inhibition in HS remains investigational. Clinicians should continue to rely on established treatment guidelines, including biologic therapies targeting TNF-α and other immunomodulatory pathways, while monitoring developments from ongoing clinical trials such as LOTUS.
References
- Avalo Therapeutics announces completion of enrollment in phase 2 LOTUS trial of AVTX-009 for the treatment of hidradenitis suppurativa. News release. Avalo Therapeutics. Published October 29, 2025. Accessed October 29, 2025.
https://www.globenewswire.com/news-release/2025/10/29/3176254/0/en/Avalo-Therapeutics-Announces-Completion-of-Enrollment-in-Phase-2-LOTUS-Trial-of-AVTX-009-for-the-Treatment-of-Hidradenitis-Suppurativa.html - Krueger JG, Frew J, Jemec GBE, et al. Hidradenitis suppurativa: new insights into disease mechanisms and an evolving treatment landscape. Br J Dermatol. 2024;190(2):149-162. doi:10.1093/bjd/ljad345
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.