
In this table, we highlight some of the forthcoming changes to the Medicare Physician Fee Schedule (PFS) from the Centers for Medicare & Medicaid Services (CMS).

In this table, we highlight some of the forthcoming changes to the Medicare Physician Fee Schedule (PFS) from the Centers for Medicare & Medicaid Services (CMS).

Outpatient dermatology services can be available to any patient. However, according to recent research, that doesn’t mean all patients access or take advantage of this clinical care in the same way.

Robot dermatologists aren’t yet seeing patients for routine care, but artificial intelligence (AI) and other technology tools are growing in popularity throughout the industry. Learn more about how these advances in technology are impacting practices.
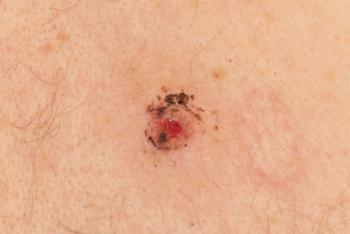
Treatment options for metastatic melanoma have greatly improved, including in the adjuvant setting, and these advances are reflected in the recently updated American Academy of Dermatology clinical guidelines on the management of primary cutaneous melanoma. Find out what has changed in this article.

Artificial intelligence (AI) tools are steadily growing in popularity throughout medicine, particularly in dermatology. But does AI pose a threat to dermatologists? Find out in this article.

In this article, Katja Reuter, Ph.D. critiques a study by Katz and colleagues on the effectiveness of using digital platforms to recruit for an atopic dermatitis study.

Do you know the warning signs of life threatening purpura? Dr. Roderick Hay outlines what you need to know about potentially life-threatening conditions involving purpura.

Digital recruitment for clinical studies may be more effective than traditional methods, according to a new study.

What exactly is digital health? Dr. Steve Xu tackles this question in this month's Innovation column.

In this article, we take a look at the future of dermatology, including the rise of medical devices and drug costs.

The FDA has reversed a long-standing guidance stating that sponsors of clinical trials for atopic dermatitis (AD) in pediatrics need not start the trial in adults first. Here's what you need to know.
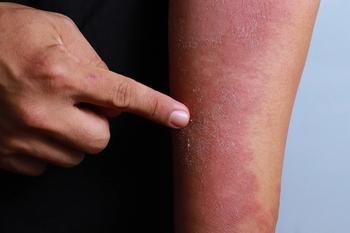
Asana BioSciences announced that the U.S. Food and Drug Administration granted fast-track designation for the investigational ASN002, an oral treatment for moderate-to-severe atopic dermatitis. Learn more in this article.

Sandoz, a division of Novartis, announced that the U.S. Food and Drug Administration approved its biosimilar, adalimumabadaz (Hyrimoz, Novartis AG).
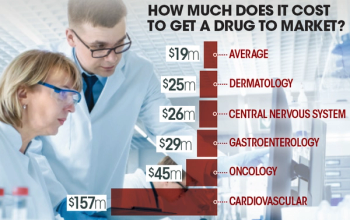
The cost of clinical trials to get a dermatology drug to market may cost as much as $25 million, which is slightly above a median of $19 million, according to the latest research.

The American Academy of Dermatology annual meeting will be held from March 1-5 in Washington, D.C.

The National Psoriasis Foundation and the National Eczema Association have awarded Procter & Gamble’s Tide Free and Gentle Liquid Laundry Detergent and Tide PODS Free and Gentle Laundry Detergent a “Seal of Recognition” and “Seal of Acceptance” for people with eczema or sensitive skin.

When can a physician disclose protected health care information? Dr. David J. Goldberg addresses this question in this month's Legal Eagle column.

In this article, Dr. Ronald G. Wheeland offers his point of view on how to define success in dermatology.

In this month's Cosmetic Conundrums, Dr. Draelos explains cleanser mildness, the chemical characteristics of mild cleansers and how these cleansers are tested.