
Miller, Vice President, Immunodermatology Disease Area Leader, Johnson & Johnson, spoke with Dermatology Times about recently published data supporting JNJ-2113's potential in this indication.

Miller, Vice President, Immunodermatology Disease Area Leader, Johnson & Johnson, spoke with Dermatology Times about recently published data supporting JNJ-2113's potential in this indication.

Almirall now possesses the rights to develop and commercialize NN-8828, particularly in immune inflammatory dermatological diseases.

Keep up with the latest headlines in dermatology from the past week, including the commercialization of a skin cancer diagnostic AI in South Korea, cannabis extract's potential in combatting melanoma cells, and more.

The combination therapy did not yield any effect in patients with non-segmental vitiligo and prompts additional large studies prior to conclusive efficacy.

The IFPA Forum Roadmap for Asia outlines key advocacy demands and practical strategies to improve psoriatic care throughout the continent.
We want to know your thoughts on the recent guidelines. Share with us by answering our poll or emailing us at DTEditor@mmhgroup.com.

Among the many sessions to be held at the Winter Clinical Miami 2024 Conference in Miami Beach, Florida, several Dermatology Times Editorial Advisory Board members are preparing to share their knowledge and insights.

Click here to answer our poll about the upcoming conference.
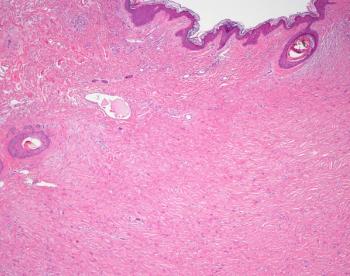
A case report examined an incidence of storiform collagenoma, also known as sclerotic fibroma, associated with Cowden syndrome in a patient. This occurrence is considered rare.

Researchers noted that this study's findings are consistent with prior epidemiologic data.

Click here to answer our poll about the upcoming conference in Puerto Rico.
The resubmission follows the Complete Response Letter Citius received from the US FDA in July of 2023.

Artax announced that safety and efficacy data is expected in the second half of the year.

A recent study revealed improvements in sensitivity but highlighted disparities in diagnostic accuracy, particularly among non-specialists and in darker skin tones.

Alongside expert dermatologists, the NRS recently developed a Seal of Acceptance for skin care and cosmetic products clinically tested and evaluated for patients with rosacea.

Keep up with the latest headlines in dermatology from the past week, including biotech's potential role in alleviating symptoms of skin disease, new sun safety guidelines in Australia, and more.

The Skin Cancer Foundation’s initiative, Destination Healthy Skin, travels to various cities throughout the country in an RV, wherein volunteer dermatologists provide on-the-street, free-of-cost skin screenings.

Both cream options were capable of reducing scores of itching, drying, burning, redness, and more.

Interim data from the clinical trial was presented in a poster at the South Beach Symposium, with itch reduction demonstrating immediacy and long-term capability.

The partnership is focused on exploring innovative therapeutic strategies to address AD.

Data from the FRONTIER 1 clinical trial were recently published in the New England Journal of Medicine.
A team of dermatology physician assistants recently reviewed the current and future potential of genomic testing in the realm of dermatology.

The partnership and newly-established center will develop dermatological research and delve into skin aging, skin allergies, and inflammatory or eczematous conditions.

The Technology Breakthrough designation will enable health care providers to access exclusive pricing and terms for the solution in pediatric burn care.

Keep up with the latest headlines in dermatology from the past week, including the role of the US in hosting more than half of the world's skin cancer-related trials, new AAD guidelines for acne, and more.

Today is Women Physicians Day. We are celebrating women on our board who make positive strides in dermatology and strive to advance patient care.

ICYMI, this week we had news about VENT-03's potential as a small molecule therapeutic in dermatology, a permanent J-code for the first and only FDA-approved treatment for molluscum contagiosum, and more.

Compared with cartridge razors, safety razors demonstrated a lower incidence of erythema.
LC-OCT demonstrated higher sensitivity and negative predictive value compared to other methods of diagnosis and detection.

Dermatology Times asked our readers to share what conferences they are looking forward to in the first quarter of 2024.