Photodynamic Therapy for sBCC Advances to FDA Review
Key Takeaways
- FDA acceptance of the sNDA for Ameluz plus RhodoLED in sBCC sets a September 28, 2026 PDUFA decision date and indicates no filing deficiencies were identified.
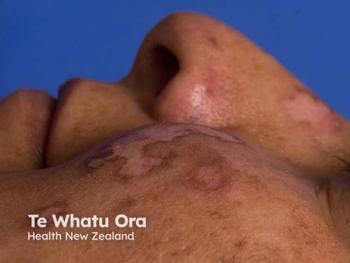
- Superficial BCC comprises an estimated 10%–25% of ~3.6 million annual US BCC cases, typically presenting as well-demarcated erythematous scaly plaques, often on trunk/extremities.
The FDA has accepted Biofrontera’s sNDA seeking approval of Ameluz PDT for sBCC, with a decision expected by September 28.
This week, Biofrontera announced that the US FDA has accepted for review its supplemental New Drug Application (sNDA) seeking to expand the indication of aminolevulinic acid hydrochloride topical gel (Ameluz), used in combination with the RhodoLED red-light lamp series, to include treatment of superficial basal cell carcinoma (sBCC). The FDA reported no filing deficiencies and assigned a Prescription Drug User Fee Act (PDUFA) target action date of September 28, 2026.1
For clinicians, FDA acceptance of an sNDA signals that the agency has determined the application is sufficiently complete to permit substantive review. While it does not imply approval, the absence of filing deficiencies suggests that the submitted clinical and manufacturing data met initial regulatory standards. The upcoming review will determine whether the therapy’s established photodynamic platform can be formally extended beyond its current US approval for actinic keratosis (AK) to a subset of non-melanoma skin cancer.2
Basal cell carcinoma is the most common malignancy in the United States, with approximately 3.6 million cases diagnosed annually. Estimates indicate that 10% to 25% of these cases represent the superficial subtype. Superficial BCCs are typically characterized by erythematous, scaly plaques with well-demarcated borders and are most often found on the trunk and extremities, although they may occur elsewhere. While rarely metastatic, BCC can cause significant local tissue destruction if inadequately treated.
Standard management of sBCC generally centers on surgical approaches, including excision and Mohs micrographic surgery, both of which offer high cure rates. Destructive techniques such as curettage and electrodessication, cryotherapy, and, in selected cases, topical therapies (e.g., imiquimod or 5-fluorouracil) are also used. The choice of therapy is influenced by lesion size, location, histologic subtype, patient comorbidities, cosmetic considerations, and patient preference. Although surgery remains the gold standard for many patients, non-invasive options are often considered when cosmetic outcomes, field cancerization, medical comorbidities, or patient reluctance toward surgery are central concerns.3
Photodynamic therapy (PDT) has long held a role in dermatology, particularly in the treatment of actinic keratoses and certain superficial non-melanoma skin cancers outside the United States. The approach combines a topical photosensitizing agent with visible light exposure to generate reactive oxygen species that selectively destroy targeted cells. Biofrontera’s therapy is formulated as a nanoemulsion of aminolevulinic acid (ALA), designed to enhance penetration into the epidermis and potentially into more superficial dermal layers. After topical application and incubation, exposure to red light from the RhodoLED lamp activates accumulated protoporphyrin IX within dysplastic or neoplastic cells, inducing cytotoxic effects.
One distinguishing feature of this platform is its use of red light, which penetrates deeper into tissue than shorter wavelengths such as blue or green light. In theory, this may support treatment of lesions extending beyond the most superficial epidermal layers—an important consideration in sBCC, where tumor nests can involve the superficial dermis. Whether this theoretical advantage translates into clinically meaningful differences in clearance rates, recurrence risk, or cosmetic outcomes in the US population will be central to the FDA’s review.
If approved for sBCC, this therapeutic combination would represent a significant expansion of the product’s labeled use. Currently approved in the US for the treatment of actinic keratosis, the drug-device combination is already familiar to many dermatology practices. An additional indication for superficial BCC could offer clinicians a non-surgical option within an existing procedural framework. In practical terms, this may be particularly relevant for patients with multiple lesions, lesions in cosmetically sensitive areas, or those who are poor surgical candidates.
However, clinicians will likely weigh several considerations. Clearance rates and long-term recurrence data relative to surgical standards remain critical benchmarks. Treatment tolerability, including procedural pain, erythema, crusting, and post-inflammatory pigmentary changes, also factors into patient selection and counseling. Reimbursement logistics and in-office workflow requirements may further influence adoption.
Biofrontera has framed the sNDA submission as part of a broader strategy to expand the clinical utility of its PDT platform, including ongoing investigations in non-melanoma skin cancers and moderate to severe acne. From a practice perspective, expansion into sBCC could modestly shift the therapeutic landscape, particularly for carefully selected low-risk lesions.
The FDA’s target action date of September 28, 2026, sets the timeline for regulatory decision-making. Until then, clinicians will await detailed efficacy and safety data to assess where, and for whom, this modality may best fit within established treatment algorithms for sBCC.
References
- Biofrontera announces FDA filing acceptance of supplemental new drug application for Ameluz® PDT in superficial basal cell carcinoma. News release. Biofrontera Inc. Published February 11, 2026. Accessed February 12, 2026.
https://www.biospace.com/press-releases/biofrontera-announces-fda-filing-acceptance-of-supplemental-new-drug-application-for-ameluz-pdt-in-superficial-basal-cell-carcinoma - FDA approves use of up to three tubes of Biofrontera Inc.’s ameluz (aminolevulinic acid HCI) topical gel, 10% in one treatment. News Release. Global Newswire. Published October 7, 2024. Accessed February 12, 2026.
https://www.globenewswire.com/en/news-release/2024/10/07/2958931/0/en/FDA-Approves-Use-of-Up-To-Three-Tubes-of-Biofrontera-Inc-s-Ameluz-aminolevulinic-acid-HCI-Topical-Gel-10-In-One-Treatment.html - Ran Zhu T, Islam Z, Chahine A, Manning J, Ciocon D. Medical and surgical management of multifocal superficial basal cell carcinoma. J Drugs Dermatol. 2025;24(5):483-488. doi:10.36849/JDD.8543
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.