New Analysis Clarifies the Dermatologic Risks of GLP-1 RA Therapy
Key Takeaways
- GLP-1 RAs are linked to increased injection-site reactions, with albiglutide showing higher rates, but reactions are typically mild and transient.
- Broader dermatologic effects, such as rash and urticaria, are rare with GLP-1 RAs, providing reassurance for prescribers.
Explore the latest findings on GLP-1 RA injection-site and dermatologic reactions, enhancing treatment adherence and patient counseling strategies.
As the use of glucagon-like peptide-1 receptor agonists (GLP-1 RAs) accelerates, dermatologic adverse events and injection-site reactions have become increasingly relevant to clinicians managing treatment tolerability and adherence. A new study has provided the most comprehensive evaluation to date of dermatologic and injection-site safety associated with GLP-1 RAs by integrating evidence from randomized controlled trials and real-world pharmacovigilance data.1
Background and Study Design
The most frequently reported adverse events with GLP-1 RAs are gastrointestinal, particularly nausea, vomiting, and diarrhea. These occur in approximately 30 to 60% of GLP-1 RA patients.2 Gallbladder-related adverse effects (cholecystitis, cholelithiasis, and biliary obstruction) are also prominent. Dermatologic reactions, however, accounted for 6.08% of all GLP-1 RA-related adverse events reported to the FDA, as of January 2024.3
In this systematic review, researchers conducted a systematic review and random-effects meta-analysis of trials published through December 2024, alongside a disproportionality analysis of the US Food and Drug Administration Adverse Event Reporting System (FAERS). The literature search was conducted on PubMed, Embase, and Web of Science databases and evaluated GLP-1 RA monotherapies, including dulaglutide, exenatide, liraglutide, lixisenatide, and semaglutide. After the screening process, 38 randomized controlled trials were chosen for the final analysis.
Frequency and Clinical Characteristics of Injection-Site Reactions
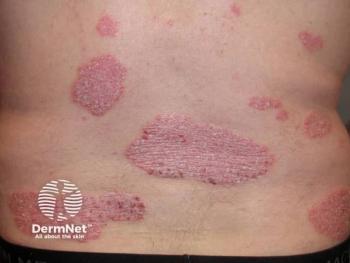
In 4,861 patients with 396 injection-site reactions, GLP-1 RAs were associated with a significantly increased risk of injection-site reactions compared with non–GLP-1 comparators. These occurred in approximately 8.1% of patients receiving GLP-1 RAs versus 2.3% of comparators, corresponding to an absolute risk difference of 5.9%. Participants had a mean age ranging from 49 to 63.5 years across treatment arms and most of the trials had a slightly higher proportion of male participants.
Furthermore, albiglutide demonstrated consistently higher adverse event rates across the studies, with incidences ranging from 8% to 28.6%. Importantly, most injection-site reactions were mild, localized, and transient, typically presenting as erythema, pruritus, bruising, or small nodules at the injection site that lasted less than 2 weeks. Treatment discontinuations were uncommon, occurring in only 1.4% of patients across all trials, underscoring that while frequent, these reactions rarely necessitate stopping therapy.
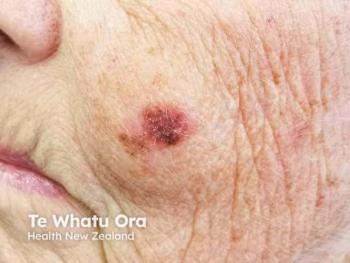
In contrast to injection-site reactions, broader dermatologic effects, including rash, pruritus, urticaria, hypersensitivity reactions, and nodules, were infrequent. Events such as generalized rash or urticaria occurred in fewer than 1% of treated patients, with no meaningful difference from comparator groups. Hypersensitivity reactions were particularly rare, with no signal of increased risk across trials. These findings suggest that GLP-1 RAs do not meaningfully elevate the risk of systemic or generalized cutaneous adverse events, a key reassurance for dermatologists and prescribers.
Real-World Evidence on Drug-Specific Signals
The FAERS disproportionality analysis revealed notable differences among individual GLP-1 RAs. Exenatide and dulaglutide demonstrated strong and consistent signals for multiple reaction categories, including injection-site hemorrhage, bruising, mass formation, pain, and injury. Exenatide, in particular, showed markedly elevated reporting odds for injection-site hemorrhage and nodules. Dulaglutide also demonstrated disproportionate reporting for several ISR types, although absolute ISR rates in clinical trials were lower.
By contrast, liraglutide, lixisenatide, and semaglutide showed fewer and weaker signals, aligning with lower event rates observed. Importantly, no GLP-1 RA demonstrated a consistent disproportionality signal for generalized dermatologic reactions such as rash, alopecia, angioedema, or urticaria in real-world reporting.
Clinical Implications and Future Directions
For dermatologists, these findings clarify that the primary cutaneous liability of GLP-1 RAs is localized rather than systemic. Awareness of agent-specific adverse event profiles—particularly with exenatide and dulaglutide—can inform patient counseling and expectation setting, which is critical to maintaining adherence. The rarity of generalized dermatologic reactions should also help reduce unnecessary discontinuation or dermatology referrals driven by concern for systemic drug eruptions.
“Future studies should focus on direct mechanistic evaluation of depot-related local immune responses and pragmatic interventions to reduce injection-side reaction incidence,” the study authors concluded.
References
1. Taj S, Zuber M, Rashid M, et al. Injection-site and dermatologic reactions associated with glucagon-like peptide-1 receptor agonists: Insights from meta-analysis of randomised controlled trials and real-world evidence. Diabetes Obes Metab. Published online December 15, 2025. doi:10.1111/dom.70382
2. Bettge K, Kahle M, Abd El Aziz MS, Meier JJ, Nauck MA. Occurrence of nausea, vomiting and diarrhoea reported as adverse events in clinical trials studying glucagon-like peptide-1 receptor agonists: A systematic analysis of published clinical trials. Diabetes Obes Metab. 2017;19(3):336-347. doi:10.1111/dom.12824
3. Ituarte BE, Taylor MA, Thomas SI, et al. Cross-Sectional Analysis of Adverse Dermatologic Events Reported to the FDA After Use of GLP-1 Agonists. J Drugs Dermatol. 2024;23(9):e181-e182.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.