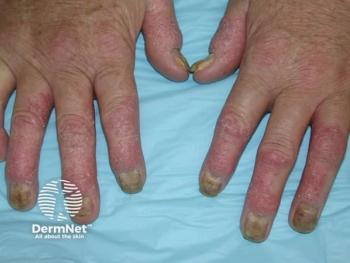
According to a poster from AAD, the National Psoriasis Foundation’s treatment goals were used as a benchmark to determine the risk of developing psoriatic arthritis after initiating biologics.

According to a poster from AAD, the National Psoriasis Foundation’s treatment goals were used as a benchmark to determine the risk of developing psoriatic arthritis after initiating biologics.

Catch up on coverage from the third day of the 2025 American Academy of Dermatology Annual Meeting held in Orlando, Florida.

Gold emphasized the necessity of recognizing and managing complications in aesthetic dermatology to ensure patient safety and optimal outcomes.

AbbVie’s phase 2 study demonstrates how upadacitinib reduces type 1 inflammation and boosts melanocyte biomarkers to drive vitiligo response.
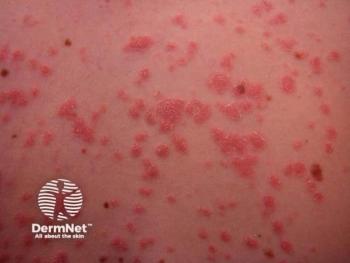
Findings suggest that most patients on risankizumab met or exceeded the National Psoriasis Foundation’s treatment goals.

Adamson dives deep into artificial intelligence, molecular testing, and melanoma overdiagnosis.

At AAD 2025, Raj Chovatiya, MD, PhD, MSCI, explored the role of social determinants in AD, HS, and PsO, emphasizing the need for improved clinical recognition and inclusive research.

Anabela Cardoso, MD, provides data on the phase 3 BRAVE-AA-PEDS trial, the largest JAK inhibitor study dedicated to adolescents with severe alopecia areata, at AAD.

Catch up on coverage from the second day of the 2025 American Academy of Dermatology Annual Meeting held in Orlando, Florida.

April Armstrong, MD, MPH, FAAD, discusses her late-breaking presentation at AAD on the treatment response in patients with moderate to severe chronic CHE treated with delgocitinib.

Late breaking data points to positive data for dupilumab for bullous pemphigoid.

Adamson highlighted melanoma's rising incidence and the growing concerns of overdiagnosis at AAD 2025.

Shawn Kwatra, MD, FAAD, reviews his late-breaking data presentation at AAD on the first original studies of a topical cream for prurigo nodularis.

Linda Stein Gold, MD, FAAD, discusses the late-breaking tapinarof cream data presented at AAD 2025.

The ADORING 3 study demonstrated that tapinarof cream 1% maintains low disease activity in patients with AD for an average of 79.8 days post-treatment.

At AAD 2025, UCB will present 5-year data for Bimzelx in psoriasis, highlighting rapid, durable responses and long-term safety for patients.

In case you missed it, this week we had news about the achievements of female dermatologists for Women’s History Month, Takeda’s initiative to improve clinical disparities in psoriasis research, previews of this week’s AAD Annual Meeting, and more.

Catch up on coverage from the first day of the 2025 American Academy of Dermatology Annual Meeting held in Orlando, Florida.

Raj Chovatiya, MD, PhD, MSCI, discusses how early-career dermatologists can gain the most benefit from this year’s annual meeting.

Jeanine Downie, MD, FAAD, discussed critical updates on tattoo ink safety, new dermatologic technologies, biostimulators, and more at AAD 2025.

The phase 2 trial of bimiralisib gel shows promising efficacy and safety for actinic keratosis, with up to 70% lesion clearance.

In addition to Taylor, several other new leaders have been appointed to key roles within the AAD to help drive its mission of advancing dermatology.

Along with his session insights on diet and chronic spontaneous urticaria, Friedman shares tips for navigating the largest dermatology conference in the US.

Ahead of the annual meeting, Dermatology Times spoke with Draelos about why she looks forward to the meeting each year.

Adamson highlights key topics to expect at the AAD Annual Meeting, emphasizing the importance of tackling controversial issues and fostering collaboration.

The company showcased advancements in tirbanibulin and its early-stage antibody LAD191.

Published in JID, the study analyzes data from the FDA’s FAERS database to examine cases of neoplasms—including skin and breast cancers—potentially associated with benzoyl peroxide use.

Read more on how to navigate all the opportunities at this year’s AAD meeting, which starts today in Orlando, Florida.

The analyzed dataset included over 200,000 patients with vitiligo to find trends in diagnosis, treatment, and more.

The findings of the MVOR-1 and MVOR-2 studies are published in JAMA Dermatology, supporting the rosacea drug’s recent FDA approval.