
Treatment is not mandatory and, in fact, clinicians are still without an FDA-approved treatment option, but parents often insist on some sort of action.

Treatment is not mandatory and, in fact, clinicians are still without an FDA-approved treatment option, but parents often insist on some sort of action.

Topicals, orals, biologics—Armstrong’s Winter Clinical Miami presentation focused on new treatment options for patients with psoriasis with an emphasis on real-world observations.

Alexandra Golant, MD, and Seemal Desai, MD, weigh the pros and cons of each treatment option for atopic dermatitis, considering mechanism of action, efficacy, and safety. Which will emerge victorious?

“So much new in the world of pediatric dermatology—new and novel treatments for atopic dermatitis, hemangiomas, childhood blistering diseases, warts. We are practicing during the Golden Age of dermatology,” Swanson says.

The proportion of patients who achieved a SALT score < 10 jumped from 24.9% at 36 weeks to 28.9% at 52 weeks in the BARI 4-mg arm and 11.8% to 15.3% in the BARI 2-mg arm.

When evaluating treatment options for patients with psoriasis, rosacea, or eczema, clinicians should employ a top-down approach and remember to consider topical therapies.

Five days of treatment with the topical therapy measurably improved symptoms and emotional/functional impact for patients with actinic keratosis at week 8.

“We're going to talk about medical hair loss in terms of diagnosis and treatment and really try to make it very easy,” Leavitt says.

From deucravacitinib to roflumilast to tralokinumab and everything in between, James Del Rosso, DO, previews his upcoming Winter Clinical Miami session on new therapies.

“It's always a fun, vibrant educational session. We learn tips and advice, and it really is for any injector, both novice [and] advanced. You learn a lot of information,” Ablon says.

“A lot of our colleagues are just getting used to the new biologics for atopic dermatitis like tralokinumab and dupilumab, which have really been breakthroughs in the treatment of atopic dermatitis,” Lebwohl, one of the co-founders of the Fall and Winter Clinical meetings, says.

With an emphasis on real-world applicability and actionable pearls, clinicians gather in Miami to kick off the newest addition to the Fall and Winter Clinical meeting lineup.

This week's edition of the Mainstream Patient features stories about quality skin care products under $20, how to address skin picking, trending mushroom skin care, and more.

Mark Nestor, MD, PhD, a co-director of the Winter Clinical Miami meeting, promises attendees a hands-on learning experience with real-world applicability.

Uncombable hair syndrome (UHS) can cause distress for families, but dermatologists can help with the rare genetic disease diagnosis and management.

PDT has an advantage in that it can combine easily with other therapies, thereby increasing its effectiveness rates.
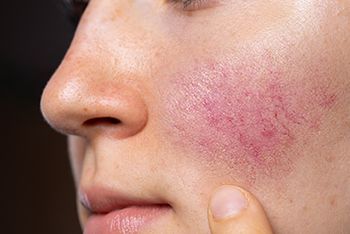
A recent study published in Clinical and Experimental Dermatology observed a link between menopause and rosacea and found that menopausal flushing can exacerbate and precipitate rosacea.

Several dietary approaches have been proposed to play a role in the pathogenesis, management, and/or therapy of diseases such as vitiligo.

In this week’s Pointers with Dr Portela, the 208SkinDoc talks to Jennifer Krejci, MD, FAAD, about preserving the hairline.

The expanded approval is based on data from a recent phase 3 clinical trial.

Discover all topics and sessions covered at South Beach Symposium.

Linda Stein Gold, MD, and Jennifer Soung, MD, discuss available nonsteroidal treatment options for plaque psoriasis in this DermView series recap.

Doctors cite more support staff, reduced patient panels among solutions.

Click here to answer this week's poll.

Aaron Farberg, MD, and Jeffrey Crowley, MD, take a closer look at the management and treatment of generalized pustular psoriasis.

Catch up on coverage from the final day of South Beach Symposium.

A poster presentation from SBS 2023 reviewed the clinician satisfaction of tirbanibulin for the treatment of actinic keratosis.

Doris Day, MD, thoroughly discussed the up-and-coming topic of the gut-skin-brain axis related to healthy skin at SBS 2023.

Lio shares key highlights of his atopic dermatitis sessions at South Beach Symposium.

Alexis shares take-home points from his popular sessions at South Beach Symposium.