
According to a poster at the 2025 EADV Congress, barzolvolimab achieved comparable improvements in weekly Urticaria Activity Scores across immunoglobulin E (lgE) subgroups.

According to a poster at the 2025 EADV Congress, barzolvolimab achieved comparable improvements in weekly Urticaria Activity Scores across immunoglobulin E (lgE) subgroups.

Sanofi's brivekimig shows promising phase 2a results for hidradenitis suppurativa (HS), offering hope for effective treatment options at EADV Congress 2025.

At EADV 2025, UCB presented new long-term data demonstrating bimekizumab’s durable efficacy and a consistent safety profile over extended treatment.

STOP-HS1 and STOP-HS2 phase 3 trials showed nearly 60% of patients achieving HiSCR50 by week 24, according to a presentation at EADV 2025.

Join Dermatology Times at the EADV Congress 2025 for expert insights and live updates from Paris, France.
By blocking 2 key inflammation signals, research presented at EADV found bimekizumab may help keep both skin and joints healthier over time.

At EADV, research showed benefits of zasocitinib on quality of life were observed as early as week 4 and sustained through week 12.

Melinda Gooderham, MSc, MD, FRCPC, emphasized the significance of the study’s results, showcasing stable laboratory parameters over a 12-week trial.

A poster presented at the EADV Congress revealed the drug's long-term efficacy in treating AD, particularly in the head and neck regions.
More than half of treatment responders maintained stable EASI ≤ 7, according to a poster from EADV.
A poster presented at EADV 2024 explored patient-reported outcomes from. the TRACE study for AD.

Shawn Kwatra, MD, presented the Incyte data at EADV 2024.

The company plans to release full 52-week OLE data for ESK-001 in early 2025, building on positive interim results.

After releasing promising data from several clinical trials, Burnett discussed the importance of studying efficacy and safety across diverse populations.
Nearly 44.2% of patients achieved HiSCR100, indicating a complete resolution of HS symptoms by the end of the 2-year period.

Arash Mostaghimi, MD, MPH, MPA, provides insights into what clinicians need to know about deuruxolitinib for severe AA.

Key highlights of the presentations included data on delgocitinib for atopic hand eczema, and tralokinumab for head and neck atopic dermatitis.

Gooderham reviews key highlights from EADV 2024 on the ADjoin long-term extension trial for patients with moderate to severe AD.

“It is critical that our clinical data represent the diversity in the world around us, and these results further reinforce our commitment to providing meaningful innovation for immune-mediated skin diseases," said Patrick Burnett, MD, PhD, FAAD, chief medical officer of Arcutis.
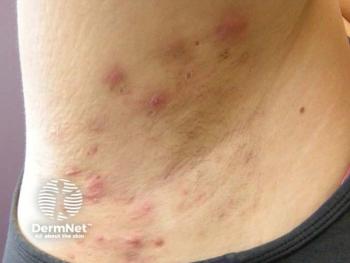
Researchers reported reductions in draining tunnels and total lesion counts.

Kilian Eyerich, MD, PhD, shares insights into an EADV 2024 presentation on upadacitinib’s ability to achieve almost total skin clearance in the head and neck regions with minimal effect on quality of life.
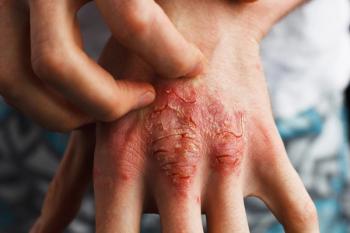
The findings stem from post-hoc analyses of previous trials, which found that 75.1% of patients reached PASI100 after 1 year on bimekizumab.

In addition to decreases in BMI, researchers also reported fewer HS flare ups.

The study found that over 80% of patients who responded to lebrikizumab at week 16 maintained clear or almost-clear skin after 3 years.
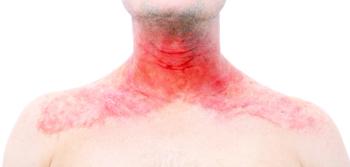
A new analysis from phase 3 studies shows Rinvoq's effectiveness for moderate to severe AD with head and neck involvement.

Kwatra presented the abrocitinib data at EADV 2023.

The expanded study data correlates with the safety and efficacy of ruxolitinib cream in patients with atopic dermatitis.
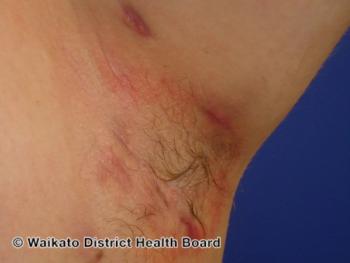
After maintenance treatment with sonelokimab dosed every 4 weeks, 57% of patients achieved HiSCR75 at week 24.
Overall, acne events were mild or moderate and did not cause patients to discontinue ruxolitinib cream treatment.

The continued reduction in WI-NRS was seen in patients who remained in the study through week 52.