At ISDS 2025, Robert Bissonnette, MD, presented new findings from a short-duration, intra-patient controlled study evaluating how quickly topical triamcinolone can elicit both clinical and molecular changes in atopic dermatitis (AD). Bissonnette, from Montreal, QC, Canada, is the CEO and medical director of Innovaderm Research and past president of the IEC. The purpose of the study was not simply to confirm the efficacy of a well-established corticosteroid but to explore whether measurable signals of activity could be detected within days, thereby reshapong how early-phase topical drug development is approached.1
Key Takeaways
• Triamcinolone acetonide 0.5% produced significant pruritus improvement within 24 hours in moderate AD lesions.
• Tape-strip transcriptomics detected early reversal of AD-associated gene dysregulation, particularly in Th2-related pathways.
• Vehicle-treated skin showed only minimal, non-significant transcriptomic movement over the same timeframe.
• The study introduces a rapid, intra-patient evaluation model that may streamline early-phase topical drug development.
• Short-term molecular readouts could help compare formulations, concentrations, and new chemical entities more efficiently.
Bissonnette underscored the broader challenge the field faces. Even though most patients with mild to moderate AD are candidates for topical therapy, innovation in this space has lagged far behind systemic therapeutics.2 As he noted, “drug development with topical therapy is not easy… with topical drugs, we have the issues of formulation and penetration.” Regulatory requirements compound these challenges: developers often need to complete healthy-volunteer tolerance studies and then multi-week, vehicle-controlled proof-of-concept trials involving dozens of patients. This traditional pathway is slow, expensive, and poorly suited to rapid screening of new chemical entities or formulations.
Methods and Materials
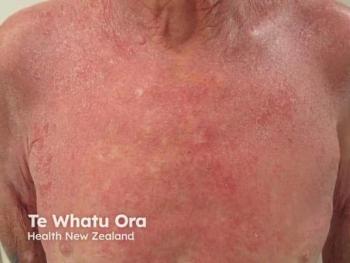
The investigators sought to create a model that would yield efficacy-relevant data within a matter of days rather than weeks. They enrolled 20 adults with moderate AD, each required to have 2 matched lesions of identical severity. One lesion received triamcinolone acetonide 0.5% cream, while the other received vehicle. To ensure dosing consistency, both were applied twice daily under staff supervision at a fixed quantity of 3 mg/cm². Clinical outcomes included regional Investigator Global Assessment, Target Area Assessment, TSS sign score, and frequent patient-reported pruritus assessments collected every 12 hours. In parallel, the team obtained tape-strip samples at baseline and again at 24, 48, and 72 hours to examine gene expression changes associated with AD inflammation and barrier dysfunction.
Findings
The clinical findings were notable for their speed. Bissonnette emphasized this point clearly: “At 24 hours, there is a significant difference in terms of change from baseline to pruritus… favoring triamcinolone.” Improvement continued to diverge at subsequent time points, and although vehicle-treated sites showed mild changes, likely reflecting moisturization, they never approached the degree of symptomatic relief observed with active treatment.
The transcriptomic data added a mechanistic dimension to these observations. The most striking result was the demonstration of rapid partial normalization of AD-related gene dysregulation. Differential expression analyses revealed that triamcinolone-treated skin exhibited significant shifts as early as 24 hours, with progressive deepening of effect at 48 and 72 hours. Pathways commonly implicated in AD—, ncluding Th1, Th2, Th17, Th22 signaling, epidermal barrier responses, and pruritus-associated transcripts, showed early and meaningful modulation. Th2-related genes, central drivers of AD inflammation, demonstrated statistically significant improvement at 24 hours. Vehicle-treated lesions exhibited small but non-significant transcriptomic movement over time, aligning with the known but modest biologic effects of occlusion and emollients.
Conclusion
These findings represent one of the clearest demonstrations that a conventional topical corticosteroid can induce both symptomatic and molecular improvement within a single day of therapy. More importantly, the work provides a potential template for accelerating the earliest phases of topical drug development. Bissonnette explained that seeing transcriptomic shifts this early “opens the possibility of doing early phase 1 studies” that can compare candidate molecules, concentrations, or vehicles with only minimal exposure and reduced regulatory burden. He discussed the feasibility of evaluating multiple formulations simultaneously within the same patient, using short-term paired comparisons to determine which iterations merit advancement to longer and costlier trials.
In clinical practice, dermatologists are familiar with the rapid relief that corticosteroids often provide. This study offers a molecular explanation for that experience and suggests a path forward for bringing new topicals to patients more quickly. The ability to capture efficacy signals within 24 to 72 hours, both at the symptomatic level and at the transcriptomic level, could substantially reshape how early topline decisions in topical drug development are made. Ultimately, the model presented here has the potential to reduce risk, accelerate innovation, and improve the alignment between mechanistic promise and clinical progression.
References
- Bissonnette R. Improvement of skin transcriptome and pruritus within 24 hours with topical triamcinolone in atopic dermatitis: A randomized, vehicle-controlled study. Presented at: Inflammatory Skin Disease Summit 2025; November 12-15, 2025; New York, New York.
- Lazar M, Zhang AD, Vashi NA. Topical treatments in atopic dermatitis: An expansive review. J Clin Med. 2024;13(8):2185. Published 2024 Apr 10. doi:10.3390/jcm13082185