Lebrikizumab Enters Late-Stage Testing for Nummular Eczema
Key Takeaways
- Nummular eczema is a chronic inflammatory skin disorder often misdiagnosed, leading to management delays and significant quality of life impact.
- Standard treatments for nummular eczema are often insufficient, with no approved systemic or biologic therapies, highlighting a significant therapeutic gap.
Almirall initiates a pivotal phase 3 trial for lebrikizumab, targeting nummular eczema and aiming to enhance treatment options for this challenging condition.
Almirall has announced plans to initiate a phase 3 clinical trial evaluating lebrikizumab for the treatment of nummular eczema, marking a notable development in an area where therapeutic options remain limited.1 Although lebrikizumab is already approved for moderate to severe atopic dermatitis, this study represents one of the most advanced attempts to formally assess a targeted biologic therapy in nummular eczema, a condition that is often challenging to manage in routine clinical practice.2
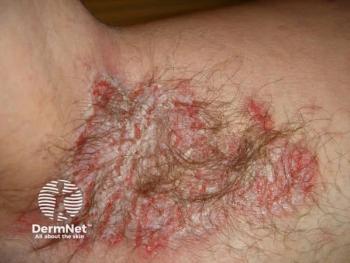
Nummular eczema, also known as discoid eczema, is a chronic inflammatory skin disorder characterized by well-demarcated, coin-shaped plaques that are typically erythematous, scaly, and intensely pruritic. While the morphology is distinctive, the condition is frequently misdiagnosed as psoriasis, tinea corporis, or other eczematous dermatoses, leading to delays in effective management. Prevalence estimates vary widely, ranging from 0.1% to 9%, reflecting differences in diagnostic criteria, age distribution, and population studied. It is more commonly reported in older adults and certain ethnic groups, and its relapsing course can have a substantial impact on quality of life through persistent itch, sleep disturbance, and visible skin lesions.
Clinicians have long recognized that standard treatments, primarily topical corticosteroids and emollients, are often insufficient for a subset of patients. Some individuals experience incomplete responses, frequent relapses, or adverse effects that limit long-term topical therapy. Unlike atopic dermatitis, there are no approved systemic or biologic therapies specifically indicated for nummular eczema, and off-label approaches are frequently used when disease is refractory. This therapeutic gap has fueled interest in better defining the underlying biology of the condition.
Although nummular eczema is distinct from atopic dermatitis, emerging evidence suggests overlapping immunologic pathways. In particular, type 2 inflammation appears to play a role in at least a proportion of patients. Recent translational studies have pointed to interleukin-13 (IL-13) as a key cytokine involved in driving inflammation, pruritus, eosinophilic infiltration, and skin barrier dysfunction in nummular eczema. These findings provide a mechanistic rationale for exploring IL-13–targeted therapies beyond atopic dermatitis.
Lebrikizumab is a monoclonal antibody with high affinity and selectivity for IL-13. By preventing IL-13 from signaling through its receptor complex, lebrikizumab interrupts downstream inflammatory pathways implicated in skin barrier impairment and itch. In atopic dermatitis, this mechanism has translated into clinically meaningful improvements in disease severity, pruritus, and quality of life, with a safety profile that has supported its approval in both Europe and the United States. Whether similar benefits can be achieved in nummular eczema remains an open but scientifically plausible question.
The upcoming LumiNE trial is designed to address this question rigorously. It is a phase III, randomized, double-blind, placebo-controlled, multicenter study that will enroll approximately 270 adults with nummular eczema across around 60 European centers, with the possibility of expansion. Eligible participants will have disease that is inadequately controlled with topical corticosteroids or for whom such treatment is not advisable, reflecting a population commonly encountered in specialist practice. Treatment is planned for up to 48 weeks, including a double-blind extension, allowing for assessment of both short- and longer-term outcomes.
The primary endpoint of the study is improvement in the Investigator’s Global Assessment for nummular eczema (IGA-NE), a clinically relevant measure of overall disease severity. Key secondary endpoints include changes in pruritus severity, assessed by a numerical rating scale, and changes in the Dermatology Life Quality Index. Together, these endpoints aim to capture not only visible skin improvement but also symptoms and patient-reported impact, which are central to the burden of the disease.
From a clinical perspective, the LumiNE study is notable for formally recognizing nummular eczema as a condition that may warrant targeted, mechanism-based therapy rather than extrapolation from atopic dermatitis management alone. At the same time, important questions remain. Nummular eczema is a heterogeneous condition, and it is unclear whether IL-13–driven inflammation is equally relevant across all patients or disease phenotypes. The extent to which trial results will translate to real-world practice will depend on both efficacy outcomes and tolerability over extended treatment periods.
If successful, this study could help clarify the role of IL-13 in nummular eczema and potentially expand the therapeutic landscape for a condition with significant unmet need. Until results are available, lebrikizumab’s role in nummular eczema should be viewed as investigational, but the trial reflects a broader trend toward biologic precision in inflammatory skin disease—one that many clinicians will be watching with interest.
References
- Almirall announces “LumiNE”, a phase III clinical study assessing the efficacy of lebrikizumab for the treatment of nummular eczema. News release. Almirall. Published January 15, 2026. Accessed January 15, 2026.
https://www.businesswire.com/news/home/20260114791875/en/Almirall-Announces-LumiNE-a-Phase-III-Clinical-Study-Assessing-the-Efficacy-of-Lebrikizumab-for-the-Treatment-of-Nummular-Eczema - Leung AKC, Lam JM, Leong KF, Leung AAM, Wong AHC, Hon KL. Nummular eczema: An updated review. Recent Pat Inflamm Allergy Drug Discov. 2020;14(2):146-155. doi:10.2174/1872213X14666200810152246
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.
.png)