- Dermatology Times, August 2019 (Vol. 39, No. 8)
- Volume 39
- Issue No. 8
EC moves forward with new PsA treatment
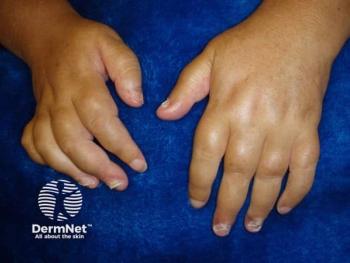
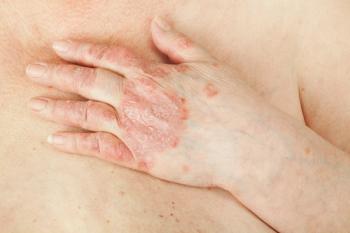
In June, the European Commission approved tofacitinib citrate (Xeljanz/Pfizer) 5 mg twice daily in combination with methotrexate for adults with psoriatic arthritis. Learn about the parameters here.
In June, the European Commission approved tofacitinib citrate (Xeljanz/Pfizer) 5 mg twice daily in combination with methotrexate for the treatment of active psoriatic arthritis (PsA) in adult patients who have had an inadequate response or who have been intolerant to a prior disease-modifying antirheumatic drug (DMARD) therapy.
Tofacitinib citrate is the first and only oral Janus kinase (JAK) inhibitor to be approved in the European Union for adults with active PsA.
The approval was based on the results of a phase three trial called the "Oral Psoriatic Arthritis Trials (OPAL)" which included two trials: OPAL Broaden and OPAL Beyond. Results from the long-term extension trial OPAL Balance were also considered.
Articles in this issue
over 7 years ago
Communication in the era of patient-centered careover 7 years ago
Comprehensive psoriasis managementover 7 years ago
Dermatology training for nurses and PAsover 7 years ago
Is it time to regulate probiotics in cosmetics?over 7 years ago
11 steps to improving doctor-patient communicationover 7 years ago
Make patient-centered work in practiceover 7 years ago
New AAD cSCC treatment guidelinesover 7 years ago
Emotional burden of skin conditions may be rooted in physiologyover 7 years ago
Outlook for new botulinum toxins in the U.S. marketNewsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.