Del Rosso's What's New in the Medicine Chest 2026: Topicals
Key Takeaways
- Berdazimer gel 10.3% is the first FDA-approved home-use topical therapy for molluscum contagiosum, leveraging nitric oxide’s antiviral activity for lesion reduction.
- Solifenacin gel 12.45% targets primary axillary hyperhidrosis, offering a focused anticholinergic approach with meaningful reductions in sweat production and improved patient-reported outcomes.
James Del Rosso, DO, discusses innovative topical therapies for dermatologic conditions.
In a recent interview with Dermatology Times, James Del Rosso, DO, highlights an often-overlooked area of dermatologic care: newer topical therapies that address common conditions outside the current focus on biologics and systemic agents. While inflammatory diseases such as psoriasis, atopic dermatitis, hidradenitis suppurativa, vitiligo, and alopecia areata continue to dominate educational programs and clinical discussions, Del Rosso emphasizes that advances in topical therapeutics remain highly relevant to daily practice—particularly for pediatric patients and those with localized disease.
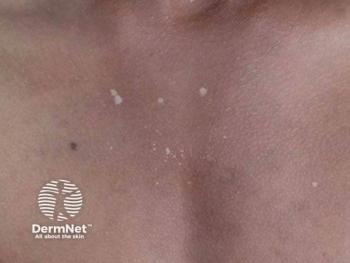
One focus of the discussion is molluscum contagiosum, a viral infection caused by a poxvirus that is especially common in children. Historically, management has ranged from watchful waiting to destructive in-office procedures, each with limitations related to tolerability, adherence, or prolonged disease duration. Del Rosso reviews the first FDA-approved topical therapy that can be applied at home: berdazimer gel 10.3%, a nitric oxide–releasing agent approved for patients one year of age and older. Applied once daily to affected areas (excluding the periocular region), the therapy leverages nitric oxide’s antiviral activity, which acts rapidly despite its very short half-life.
Clinical trial data supporting approval demonstrated a gradual but consistent reduction in lesion counts over a 12-week treatment period. Importantly, new lesions may continue to appear during therapy due to the incubation period of the virus, requiring continued application to emerging lesions. Del Rosso underscores that this pattern reflects the natural course of molluscum contagiosum rather than treatment failure. Early intervention, he notes, may help reduce overall disease duration, which can otherwise persist for a year or longer in many children. Local skin irritation remains the most commonly reported adverse effect.
Del Rosso also addresses topical management of primary axillary hyperhidrosis, highlighting solifenacin gel 12.45%, approved for patients 9 years of age and older. This anticholinergic agent is designed as a “softer,” more targeted molecule, with activity focused on muscarinic receptors involved in sweat production. Applied once nightly, the formulation is delivered via a specialized applicator that minimizes hand contact and reduces the risk of inadvertent ocular exposure, a known concern with earlier topical anticholinergics that could lead to blurred vision or pupil dilation.
Clinical studies demonstrate meaningful reductions in sweat production, accompanied by improvements in patient-reported outcomes. As Del Rosso notes, patients often experience substantial quality-of-life benefits even with partial reductions in sweating, without the need to completely eliminate physiologic perspiration.
Together, these examples reinforce an important clinical message: while systemic therapies continue to transform dermatologic care, thoughtfully designed topical treatments remain essential tools. For conditions such as molluscum contagiosum and axillary hyperhidrosis, newer topical options offer practical, patient-centered approaches that align well with real-world clinical needs.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.