
Expert calls for FDA approval of devices for women’s genitourinary health: “Women need these treatments.”

Expert calls for FDA approval of devices for women’s genitourinary health: “Women need these treatments.”

Chief executive officer of Can-Fite Biopharmaceuticals, Pnina Fishman, Ph.D. discovered why cancer and inflammation can go everywhere in the body, except muscle tissue. Using this knowledge, Can-Fite has developed a promising psoriasis drug, piclidenoson, which is currently in phase 3 clinical trials.

Leadership and representation are becoming more important as the profession and demand for PAs grow. However, a recent study found that career satisfaction does not appear to be related to professional development benefits offered by employers. What fulfills physician assistants? Participate in this forum.

Learn how the Food and Drug Administration (FDA) plays a critical role in the oversight of many medical devices used commonly in dermatology.

From lasers to light therapy to non-laser-based thermal tightening, learn each devices' indications, adverse effects and important treatment takeaways.

Drug-device combination, VP-102, met all primary and secondary endpoints in the parallel Cantharidin Application in Molluscum Patients (CAMP)-1 and CAMP-2 trials.

Psoriasis treatment has expanded dramatically over the past decade, and the growth shows no signs of stopping. Andrew Blauvelt, M.D. discussed new drugs in the pipeline during American Academy of Dermatology Annual Meeting in Washington, D.C.

To help providers have a greater understanding of the efforts of the FDA to ensure all drugs are of pharmaceutical quality, Michael Kopcha, Ph.D., RPh, director of the FDA’s Office of Pharmaceutical Quality presented on the topic during the American Academy of Dermatology’s Annual Meeting in Washington, D.C.
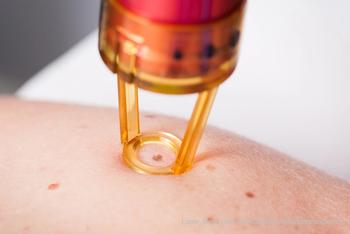
Laura Marquart, M.D., FAAD, medical officer with the FDA’s Center for Devices and Radiological Health (CDRH), reviewed how the FDA approaches, reviews, and approves medical devices integral to dermatologic care at the American Academy of Dermatology’s annual meeting in Washington, D.C. Learn how this process can affect patient care in this article.

Longer-lasting treatment for vitiligo could be available within the next few years, according to research presented at the American Academy of Dermatology Annual Meeting in Washington, D.C.

Treating chronic inflammatory skin conditions during pregnancy can be difficult because the therapies dermatologists prescribe can affect the unborn infant. See what insights this physician offers on managing chronic inflammatory skin conditions in pregnant patients.

Dr. Jacob Mashiah offers tips for optimally treating patients with sweating disease.

How was the 2019 Dermatology Hall of Fame Executive Board appointed? Find out in this article.


In this article, Joseph C English III, M.D., professor of dermatology at the University of Pittsburgh, offers his insight for diagnosing and treating cutaneous blistering diseases. Participate in this forum.

Organic and microbiome friendly cosmeceuticals are two big trends to watch out for in 2019, according to this physician.

Dermatology care for women goes beyond skin. Practicing dermatologists reside at the crux of meeting women’s needs and play an important role in addressing issues like aging, self-esteem, hormones and stress management.
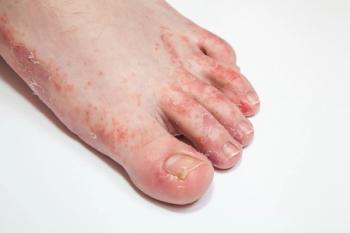
Dermatological conditions that lead to foot pain and discomfort do not necessarily require costly and time-consuming prescription management. Instead, according to a new study, over-the-counter treatments can be a viable option for patients.

For the last several decades, global incidence rates of onychomycosis have risen. This method may be an affordable and effective tool physicians can use to improve diagnosis quickly.

Looking into the future, dermatologists can anticipate new technologies and treatments to enhance services. In this article, we delve into the how these advances will affect daily workflow.

Healthcare disparities exist in all fields of medicine, including dermatology. We seek to address these disparities in this article by outlining the differences in epidemiology, presentation, access and outcomes of five conditions in patients with skin of color.

The Food and Drug Administration(FDA) approved in December a new drug application for Tolsura (SUBA-itraconazole), a new formulation of itraconazole for the treatment of systemic fungal infections in adults.

Fillers and skin tightening technologies provide safe, synergistic results.

With the recent FDA approval, Jeuveau execs believe they’ve got a brand that could neutralize the existing monopoly.

The FDA has approved STP705, an siRNA (small interfering RNA) therapeutic for in situ Squamous Cell Carcinoma Nonmelanoma Skin Cancer (NMSC), to proceed with phase two clinical trials.

Vyome Therapeutics Inc. announced in January that it secured $22 million in financing to, in part, support the development of its lead molecule, VB 1953, through phase two clinical trials for moderate to severe acne.

Industry relationships are essential for innovation in dermatology. In this month's column, Steve Xu, M.D., and Michael L. Sierra, Ph.D., provide their insight on collaborating with industry.

According to recent research, smokers are more likely than nonsmokers to have complications after undergoing cosmetic surgery on the body, versus the face or breasts.

A recent study suggests that patients suffering from psoriasis and psoriatic arthritis may have an increased risk of developing autoimmune thyroid disease.

Doctors should be vigilant about recognizing the signs and symptoms of patients who may develop ILD in order to prevent catastrophic complications, such as respiratory failure.