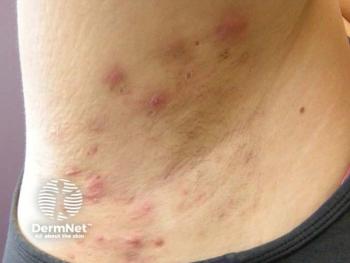
Post Hoc Analysis of Vilobelimab in Hidradenitis Suppurativa Suggests Clinically Meaningful Benefits
Researchers reported reductions in draining tunnels and total lesion counts.

Researchers reported reductions in draining tunnels and total lesion counts.

Kilian Eyerich, MD, PhD, shares insights into an EADV 2024 presentation on upadacitinib’s ability to achieve almost total skin clearance in the head and neck regions with minimal effect on quality of life.

In addition to decreases in BMI, researchers also reported fewer HS flare ups.
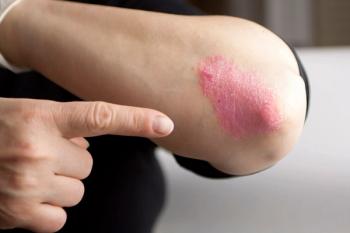
Researchers presented interim 3-year results from the European SERENA study.
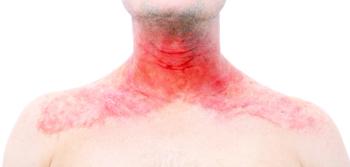
A new analysis from phase 3 studies shows Rinvoq's effectiveness for moderate to severe AD with head and neck involvement.

Farberg discusses data recently published in Geriatrics demonstrating low rates of recurrence.

VYNE recently announced positive results for its novel oral small molecule BD2-selective BET inhibitor in a single-ascending dose clinical trial.

The FDA has set a PDUFA target action date of May 22, 2025.
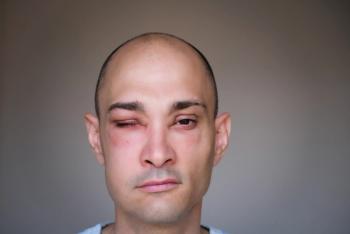
Review cutaneous signs and symptoms of HAE that may enhance early diagnosis and treatment.

Bimzelx was also approved for non-radiographic axial spondyloarthritis and ankylosing spondylitis.

Keep up with the latest headlines in dermatology from the past week, including data on AI versus dermatology residents, permanent makeup and the risk of allergic contact dermatitis, and more.

Lim discusses support for underserved communities, including dermatology training for Pacific Island Nations.

Elyse Love, MD, discusses the role of the program in supporting students with skin conditions the financial support and empowerment necessary for academic success.

Data was published in the Journal of the American Board of Family Medicine.

The Adbry 300 mg/2 mL autoinjector reduces the number of self injections required for patients, who currently have a 150mg/1mL pre-filled syringe available.

The conference aimed at providing dermatology NPs and PAs clinicians with the latest updates in the specialty begins today in Nashville, Tennessee.

Keep up with the latest headlines in dermatology from the past week, including the use of AI in a dermatology clinic to enhance gun safety, decreased rates of melanoma in Swedish adults under 50, and more.

Ebglyss is now approved for children and adults aged 12 years and older.
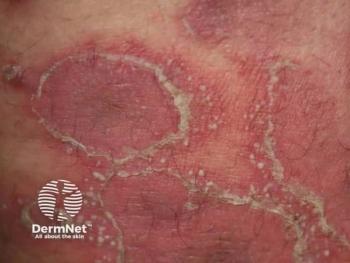
Researchers authored a manuscript detailing considerations for GPP diagnosis, definitions, and management.

Researchers reported several factors and indicators that may predict early-stage systemic sclerosis among patients in Japan.

The subsequent MAD portion of the study has commenced dosing. Results are anticipated in Q4.

Ahead of the next 3 months of the year, Dermatology Times wants to know what conferences and meetings you plan to attend between October and December. Share your thoughts with us by September 25.
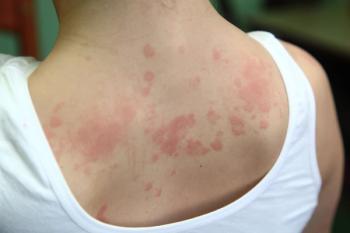
These positive data will support US regulatory submission by end of 2024.
The review assessed infections, gastrointestinal AEs, blood and lymphatic disorders, and nervous system and musculoskeletal/connective tissue disorders.

Researchers reported that second-year students were more likely to identify dermatological conditions, particularly in White patients.
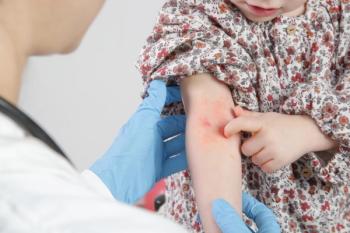
Dermatology Times spoke with Leah Howard, JD, of the NPF, and Trisha, the mother of a young girl with psoriasis, to discuss Otezla's implications in the therapeutic landscape.

Keep up with the latest headlines in dermatology from the past week, including research into "zombie cells" in skin disorders, tartrazine's effects on skin transparency, and more.
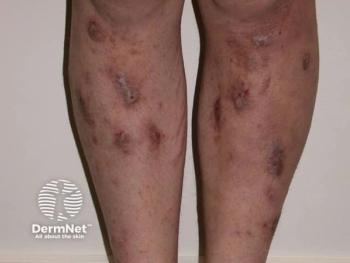
Researchers conducted a web-based survey to gain insights into current clinical practice for PN in Japan.
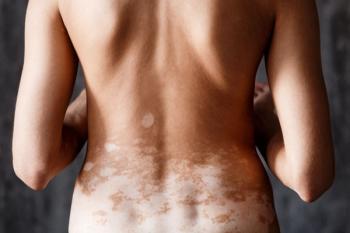
The study is actively recruiting participants at multiple sites across the US.
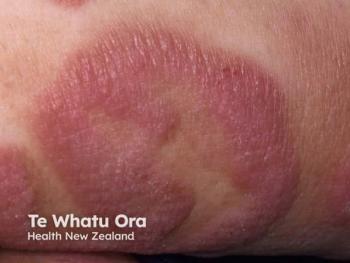
Citius' recently-approved Lymphir has been added to the National Comprehensive Cancer Network's Clinical Practice Guidelines in Oncology.