- Dermatology Times, May 2020 (Vol. 41, No. 5)
- Volume 41
- Issue 5
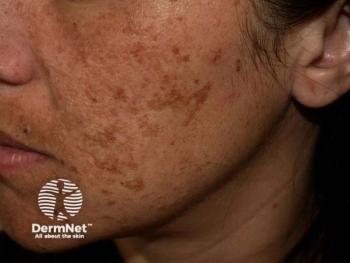
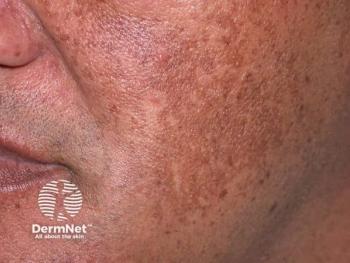
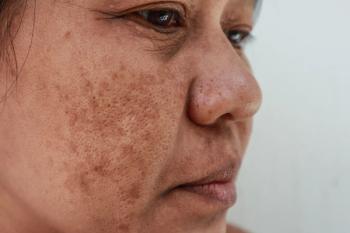
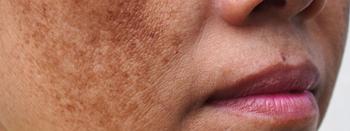
Investigational cream may be effective to deliver tranexamic acid
Melasma responds well to oral and locally injected tranexamic acid treatments, but clinical results from topical tranexamic acid administration have been disappointing, researchers report. They examined efficacy of a co-enhancer as a method to deliver the treatment.
Melasma responds well to oral and locally injected tranexamic acid treatments, but clinical results from topical tranexamic acid administration have been disappointing,
The lack of efficacy in topical preparations could be from the inability of tranexamic acid topicals to achieve epidermal therapeutic concentrations needed to lighten the pigmentary disorder, the authors write.
Researchers in Singapore conducted an in vitro study comparing human epidermal and dermal tranexamic acid skin concentrations after applying Fairence T-Complex (TDF Derma Formula), a co-enhancer cream they developed, and a Japanese branded cream as a control. Each topical had a tranexamic acid concentration of 2%.
They found tranexamic acid concentrations in the skin six and 24 hours after applying the co-enhancer cream were well within the required estimated concentration range for efficacy. They looked at interpolated concentrations at a depth of 100 µm, which is at about the dermo-epidermal junction and the therapeutic target for tranexamic acid. At six hours, interpolated concentrations for the Fairence topical were about 4 µg/mL and at 24 hours about 20 µg/mL at 100 µm tissue depth.
“A similar interpolation for Japanese branded tranexamic acid cream places 24 hour 100 µm concentrations in the lower part of the predicted efficacy range,” they write.
The co-enhancer cream showed greater skin tissue bioavailability than the control cream and achieved greater therapeutic concentrations at the viable epidermal target.
In the tissue samples studied, tranexamic acid concentrations diminished with skin depth for both formulations.
Results from this preclinical study support more research on melasma treatment with Fairence T-Complex co-enhancer cream, they write.
In the last decade, researchers have extensively studied tranexamic acid treatment for melasma. Authors of
But they note that data is limited on topical tranexamic acid, which has been applied alone and along with intradermal injection, microneedling, fractionated CO2 laser and more.
“Debate exists about whether topical water-soluble [tranexamic acid] is trans-dermally absorbed by the lipid-soluble surface of the skin. In addition to bridging the knowledge gaps in our understanding of topical as an option for melasma patients, further studies on clinical efficacy and side effects comparisons among different administrations of [tranexamic acid] should be carried out,” the review authors write.
Authors of the current study write that topical bioavailability is a concern, as it tends to be lower and more variable with topical administration than with oral tranexamic acid.
Tranexamic acid is not easy to partition into the stratum corneum barrier’s lipid domain.
“Here we formulated 2% tranexamic acid into a glycol co-enhancer drug delivery system, dispersed in silicone elastomer continuous phase, with the objective of combining pharmaceutical topical drug delivery technology with the technology of cosmetic science,” according to the authors.
While more studies, including a double-blind placebo-controlled clinical study, are needed, the authors predict the in vitro results will reflect in vivo levels. They think that by using the co-enhancer silicone cream in melasma patients to more effectively deliver tranexamic acid to the intended target, especially refractive melasma patients will achieve better treatment outcomes. The co-enhancer technology in Fairence T-Complex has been shown to increase skin penetration for other actives, they write.
Disclosures:
Disclosures: None listed on the paper.
References:
Reference: Ng, SP, Marcant, M, Davis, AF.
Articles in this issue
over 5 years ago
Medical innovation expands hair loss treatmentover 5 years ago
Medical therapy for advanced melanomaover 5 years ago
IL-23 antagonist demonstrates favorable long-term safetyover 5 years ago
Mast cells may play key role in rosacea pathogenesisover 5 years ago
The future of actinic keratosis treatmentover 5 years ago
A new target for rosacea treatment: The mast cellalmost 6 years ago
The role of ’biotics in skincarealmost 6 years ago
Practicing dermatology in the time of COVID-19almost 6 years ago
Teledermatology and HIPAA compliance in the era of COVID-19almost 6 years ago
Sebaceous gland destruction a viable strategy to treat acneNewsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.