
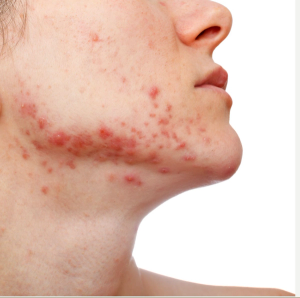
Clascoterone cream 1% phase 3 results presented at meeting.

Clascoterone cream 1% phase 3 results presented at meeting.

Ranbaxy is launching its isotretinoin product for the treatment of acne, Absorica, in the United States.

Low-dose isotretinoin may be effective, with fewer side effects, when used to treat adult acne, results of a clinical trial presented at the annual congress of the European Academy of Dermatology and Venereology suggest.

Lumenis has signed an agreement with Sound Surgical Technologies (SST) that will make Lumenis a distributor of SST’s VASER Shape MC1 System, a noninvasive body-shaping device for aesthetic practitioners.

Lucid Inc. is operating under a new company name - Caliber Imaging & Diagnostics Inc., or Caliber ID. The new name reflects the company’s commitment to innovating and delivering the highest-caliber identification tools for accurate, rapid diagnosis to assist in the fight against skin cancer and other diseases, according to company reports.

Ulthera is now registered in Brazil to market and distribute its UltheraSystem, which combines ultrasound imaging with focused ultrasound therapy in a treatment known as Ultherapy.

Valeant Pharmaceuticals has agreed to acquire Medicis Pharmaceutical for $44 per share in cash, in a deal worth approximately $2.6 billion.

Palomar Medical Technologies has announced the availability of the Palomar Vectus laser for laser hair removal.

Galderma’s Cetaphil brand is partnering with Camp Wonder, an initiative of the Children’s Skin Disease Foundation, by donating a portion of the proceeds of a special edition moisturizing cream to the camp, as well as granting $100,000 to fund camp activities.

Allergan has opened a new research and development center in Bridgewater, N.J.

NovaBay Pharmaceuticals has expanded its commercial partnership agreement with Naqu Area Pioneer Pharma, a Shanghai company that markets high-end pharmaceutical products into China, for the commercialization of NeutroPhase Skin and Wound Cleanser in China and other select Asian markets.

About 76,000 new cases of invasive melanoma occur in the United States each year, according to the American Cancer Society, and patients who have had one melanoma have a 5 to 10 percent risk of developing another primary tumor during their lifetime. As a result of this increased melanoma risk, careful follow-up of these patients is critical, a Harvard clinician advises.

Galderma Laboratories has pledged $450,000 to help support the dermatology residency program at Baylor University Medical Center at Dallas.

Sandoz has announced the introduction of its first-to-file calcipotriene cream, the first generic version of LEO Pharma’s Dovonex 0.005 percent, which is indicated for the treatment of plaque psoriasis.

Sound Surgical Technologies has announced its launch of VASERsmooth, a minimally invasive cellulite solution that works with the company’s VASER Lipo System.

ELITech Molecular Diagnostics has received FDA clearance for its MRSA/SA ELITe MGB test, a multiplex, qualitative real-time polymerase chain reaction assay for the detection of Staphylococcus aureus and methicillin-resistant Staphylococcus aureus (MRSA) DNA purified from human nasal swab specimens.

Ellman International, a developer of radiofrequency technology for precision surgical and aesthetic procedures, has announced its acquisition of Sandstone Medical Technologies, developer of aesthetic laser and intense pulsed light technologies.

RXI Pharmaceuticals has appointed Jeannette Graf, M.D., and Leroy Young, M.D., to its scientific advisory board.

Merz has announced that Katrina Church has been promoted to vice president, chief compliance officer. The appointment was effective July 1, 2012.

The Dermatology Foundation (DF) Board of Directors recently recognized the impact that Patricia G. Engasser, M.D., has had on the specialty of dermatology by presenting her with the Clark W. Finnerud Award at its annual membership meeting in San Diego.

Cohera Medical, a developer of absorbable surgical adhesives and sealants, has secured more than $8.4 million toward Series D financing through private investors, which the company reportedly will use to expand adoption of its TissuGlu Surgical Adhesive product in Germany and additional European markets.

Wellness Center USA, a healthcare and nutraceutical company, has announced the signing of a share exchange agreement to acquire Psoria-Shield, a developer and manufacturer of ultraviolet (UV) phototherapy devices for the treatment of various skin diseases.

Lumenis Ltd. announced that Roy Ramati has been appointed as the new general manager of the company’s Aesthetic Business Unit.

Verisante Technology has renewed its Collaborative Research Agreement with the BC Cancer Agency, which will include Verisante’s contribution of approximately $250,000 to fund continued development of its Aura device for skin cancer detection.

Alma Lasers has signed an agreement to acquire the assets of Quantel Derma, (formerly known as Wavelight), a France- and Germany-based developer of laser technologies for dermatologists. The transaction was expected to be finalized in July.

Merz has announced that James P. Hartman has been appointed vice president, medical dermatology, effective June 12, 2012. Mr. Hartman will be responsible for the company’s medical and OTC/OTX dermatology business unit, which includes Naftin (naftifine hydrochloride) and Mederma.

Doug Ulman, president and CEO of LIVESTRONG, has been appointed to Provectus Pharmaceuticals’ Corporate Advisory Board.

Stiefel and Welichem Biotech have entered into an agreement for Stiefel’s acquisition of exclusive development and commercialization rights to the nonsteroidal, topical anti-inflammatory agent, WBI-1001, in all territories outside of China, Taiwan, Macao and Hong Kong.

The Food and Drug Administration has delayed for six months regulations that would require sunscreen makers to change product labels to more clearly state how much protection they offer from the sun.
Published: March 28th 2022 | Updated:

Published: December 31st 2012 | Updated:

Published: December 31st 2012 | Updated:

Published: October 1st 2012 | Updated:

Published: October 1st 2012 | Updated:

Published: October 1st 2012 | Updated: