Young African American patients with psoriasis are at a higher risk of atherosclerotic cardiovascular disease (ASCVD) and traditional cardiovascular risk factors than white patients with psoriasis of the same age group, says a recent study.

Young African American patients with psoriasis are at a higher risk of atherosclerotic cardiovascular disease (ASCVD) and traditional cardiovascular risk factors than white patients with psoriasis of the same age group, says a recent study.
Laser-based procedure for autologous fat transfer may increase fat survival.

Dr. Zoe Draelos, M.D., offered skin care guidance at 2019 AAD for what to eat and how to supplement in her presentation, “Nutraceuticals: Is it Possible to Eat Your Way to Skin Health?” This is what she had to say.

Hosted by Dermatology Times and supported by Ortho Dermatologics, the hall of fame honored its inaugural class of inductees at the Long View Gallery in Washington, D.C., just before the 2019 American Academy of Dermatology Annual Meeting.

PricewaterhouseCoopers believes 2019 is the year a New Health Economy comes of age, according to its 13th annual report, "Top health industry issues of 2019." View the top six takeaways from the report in this slideshow.

New data related to consumer satisfaction trends soon to be released by RealSelf reveals the “Most Worth It” procedures for 2019.

Revance Therapeutics announced in early December that its longacting neuromodulator daxibotulinumtoxinA for Injection RT002 delivered positive results in a phase 3 study for treatment of moderate-to-severe glabellar lines.

Burt's Bees skin care products may be a effective as an adjunct to prescription therapy in the management of rosacea, according to data published in the February issue of Journal of Drugs in Dermatology which was reported at the American Academy of Dermatology Annual Meeting this month.

How was the 2019 Dermatology Hall of Fame Executive Board appointed? Find out in this article.

In this table, we highlight frequently asked questions about the staging, treatment and surveillance of early-stage melanoma.

The Dermatology Hall of Fame was established to formally honor the memory and contributions of individual physicians and the revolutionary ideas that have refined the practice of dermatology and continue to inspire, inform and improve treatment. Learn more in this article.

Mindful of all dermatology specialty areas, the Dermatology Hall of Fame nomination process is guided by specific categories and criteria, which we've detailed in this article.

A recent survey asks dermatologists who treat patients with moderate to severe psoriasis about the greatest unmet needs of this patient group. Here's what physicians had to say.

The Food and Drug Administration(FDA) approved in December a new drug application for Tolsura (SUBA-itraconazole), a new formulation of itraconazole for the treatment of systemic fungal infections in adults.

Novel therapeutic recently completed phase three CAMP-1 and CAMP-2 pivotal trials, demonstrating statistically significant improvements in patients with molluscum contagiosum.

The FDA has approved STP705, an siRNA (small interfering RNA) therapeutic for in situ Squamous Cell Carcinoma Nonmelanoma Skin Cancer (NMSC), to proceed with phase two clinical trials.

Vyome Therapeutics Inc. announced in January that it secured $22 million in financing to, in part, support the development of its lead molecule, VB 1953, through phase two clinical trials for moderate to severe acne.

This week the Dermatology Hall of Fame announced its plan to recognize its inductees in Washington, D.C. on February 28, 2019.

In this table, we highlight some of the forthcoming changes to the Medicare Physician Fee Schedule (PFS) from the Centers for Medicare & Medicaid Services (CMS).

In this article, we take a look at the future of dermatology, including the rise of medical devices and drug costs.

The FDA has reversed a long-standing guidance stating that sponsors of clinical trials for atopic dermatitis (AD) in pediatrics need not start the trial in adults first. Here's what you need to know.
Asana BioSciences announced that the U.S. Food and Drug Administration granted fast-track designation for the investigational ASN002, an oral treatment for moderate-to-severe atopic dermatitis. Learn more in this article.

Sandoz, a division of Novartis, announced that the U.S. Food and Drug Administration approved its biosimilar, adalimumabadaz (Hyrimoz, Novartis AG).
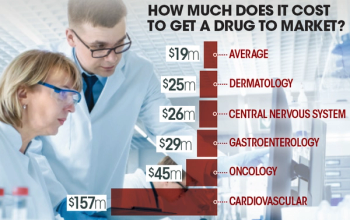
The cost of clinical trials to get a dermatology drug to market may cost as much as $25 million, which is slightly above a median of $19 million, according to the latest research.

The American Academy of Dermatology annual meeting will be held from March 1-5 in Washington, D.C.

The National Psoriasis Foundation and the National Eczema Association have awarded Procter & Gamble’s Tide Free and Gentle Liquid Laundry Detergent and Tide PODS Free and Gentle Laundry Detergent a “Seal of Recognition” and “Seal of Acceptance” for people with eczema or sensitive skin.
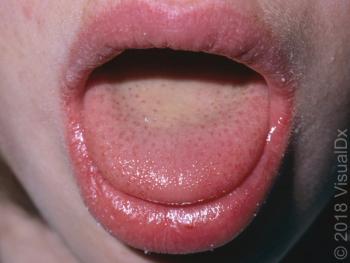
Image IQ: An 8-year-old girl with a sore throat, deep red tongue, fever and a petechial rash. What’s your diagnosis?

Before 2018 comes to a close, we wanted to say thank you to our readers from everyone here at Dermatology Times. As a thank you, we have compiled a list of the topics and issues you found most compelling throughout the year. Enjoy this slideshow - and see you in 2019!

We've gathered our latest and greatest articles on excessive sweating so you can sit back and relax over the holiday. From a new topical to Botox to treatments yet to come, our slideshow contains all you need to know about novel hyperhidrosis treatments.
