When Innovation Meets Insurance Barriers With Lisa Swanson, MD
Key Takeaways
- Epidermolysis bullosa management is expanding beyond wound care with topical therapies and a newly approved procedure that can normalize skin in treated areas.
- Neurofibromatosis type 1 now includes approved systemic therapy for unresectable plexiform neurofibromas, improving options where surgery is not feasible.
Clinicians are increasingly facing barriers when attempting to prescribe on-label treatments for indicated patients.
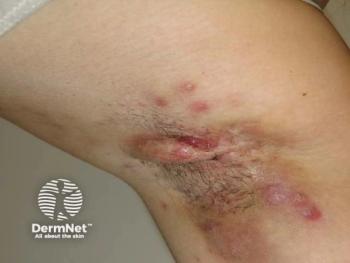
For decades, clinicians caring for patients with rare genetic skin diseases have had few therapeutic tools beyond symptom management. In a recent Dermatology Times interview at the
Swanson emphasized that epidermolysis bullosa (EB) represents one of the most striking examples of progress in recent years. “For years and years, it was all about wound care and bandages, and now we have some hope to offer our EB patients,” she said. The availability of topical therapies and a newly approved procedure capable of normalizing skin in treated areas marks a meaningful evolution in care. Swanson stressed that even clinicians who do not routinely manage EB should be aware of these advances, noting that adult patients may have affected relatives and often serve as conduits of information within families. “Just spreading the word that there's more than just wound care to be done for EB… that’s very exciting,” she added.
Similar momentum is being seen in neurofibromatosis type 1 (NF1). Swanson pointed to the approval of systemic therapies for plexiform neurofibromas that are not amenable to surgical removal, calling it “a really great thing” for patients with limited prior options. In addition, emerging data suggest a potential role for compounded topical sirolimus in the management of cutaneous neurofibromas and café-au-lait macules. While not universally effective, these topical approaches may offer cosmetic improvement for patients distressed by visible lesions, expanding the spectrum of patient-centered care.
Despite therapeutic progress, Swanson was candid about the growing challenge of insurance barriers. “The crazy thing is that I'm not having trouble getting things off-label. I'm having trouble getting things on-label,” she said. Even medications with formal FDA approval for specific age groups and indications may face repeated denials, a practice she believes is often driven by cost-containment strategies.
Swanson encouraged clinicians not to abandon appeals after an initial denial. “Don't give up! Don’t give up,” she urged, outlining a stepwise approach that includes peer-to-peer reviews, external appeals, and escalation to medical directors. If necessary, she advised filing formal complaints with state departments of insurance. Her stance is grounded in a long-held professional ethic: “The patient is number one. We need to serve our patients.”
She also addressed the operational toll of prior authorizations, describing them as “out of control.” Practical solutions, such as hiring dedicated administrative staff or leveraging third-party authorization support services, may help relieve the burden on clinicians and medical assistants, though these strategies require resources that may be challenging for smaller practices.
Taken together, Swanson’s insights reflect a dual reality in modern dermatology: unprecedented therapeutic innovation for rare diseases like EB and NF1, coupled with increasing administrative complexity. As treatment options expand, she argues, ensuring patient access must remain an equally urgent priority.
In an effort to combat the spread of misinformation on social media in 2026, Swanson created Skincredible , a podcast about skin from a credible source. The podcast was developed in response to the rapid rise of skincare content targeting tweens and adolescents, much of which promotes complex routines, anti-aging products, and skin-lightening claims without scientific support. Swanson uses the platform to address common myths circulating on social media, explain what developing skin actually needs, and help families distinguish evidence-based guidance from influencer-driven marketing.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.











