- Dermatology Times, November 2022 (Vol. 43. No. 11)
- Volume 43
- Issue 11
Epidermolysis Bullosa: The Worst Disease You’ve Never Heard Of
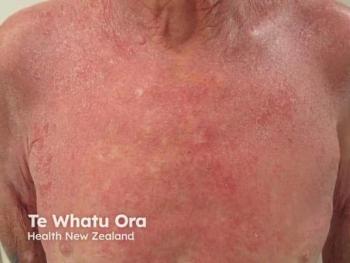
Epidermolysis bullosa is a broad term used to identify a group of genetic disorders that result in skin blistering and fragility.
Background
Epidermolysis bullosa (EB) is a broad term used to identify a group of genetic disorders that result in skin blistering and fragility. In 2020, there was a consensus for re-classification of inherited EB to include EB-related disorders such a peeling, erosive, hyperkeratotic, and connective tissue disorders.1 The focus of this brief review will be on the four main groups of EB which is differentiated by the level of skin cleavage: EB simplex (EBS), junctional EB (JEB), dystrophic EB (DEB), and Kindler EB (KEB).
Epidemiology
The National Epidermolysis Bullosa Registry (NEBR) reports, based on 16 years of data, that the incidence of EB in the United States is 19.57 per 1 million live births and the prevalence is 11.07 per 1 million population.2 Worldwide, EB impacts 500,000 lives.3 EB is a rare skin blistering disease that takes the lives of many children who suffer from these genetic defects, with the greatest risk seen in those with JEB.4
Clinical Presentation
Epidermolysis bullosa simplex (EBS)
EBS makes up 70% of overall cases encompassed by EB.3 It is also referred to as basal EBS since the level of skin cleavage occurs at the intraepidermal plane within the basal layer of keratinocytes. Various mutations have been identified that are associated with EBS, with KRT5
and KRT14 making up 75% of cases.3 Depending on the mutation, EBS has a broad spectrum of presentation ranging from minor blistering to lethal extracutaneous involvement.1 Forms of systemic involvement include nephropathy, cardiomyopathy, muscular dystrophy, and pyloric atresia.5 Kallin syndrome is a variant of EBS which presents with findings of deafness, alopecia, hypodontia, and nail dystrophy along with the classic findings of EBS.6 With 14 clinical subtypes, the majority of EBS cases in the US are mostly autosomal dominant but recessive cases have also been reported.7 Patients with EBS tend to present shortly after birth, although some may not develop blisters until later in life.8 Comparatively, EBS tends to present less aggressively than other forms of EB.
Junctional epidermolysis bullosa (JEB)
JEB is an autosomal recessive disease with early lethality affecting the lives of infants 6-24 months old.1 JEB presents with two major subtypes aptly referred to as severe and intermediate. In the US, severe JEB is the more common subtype making up 20% of all JEB cases.8 Skin blistering in JEB is characterized by skin cleavage that occurs through the lamina lucida of the cutaneous basement membrane zone or dermo-epidermal junction. Mutations associated with JEB are variable and include biallelic mutations in genes encoding laminin 332 (i.e. LAMA3, LAMB3, and LAMC2).3 Unique to all 8 subtypes of JEB is enamel hypoplasia, a useful clinical finding in patients who have erupted primary teeth.8 Pathognomonic for severe JEB is the finding of exuberant granulation tissue that might arise in the first 1-2 years of life.9 Unfortunately, JEB leads to death in early infancy and these diagnostic clues are specific but rather insensitive to the disease.9
Dystrophic epidermolysis bullosa (DEB)
DEB can be inherited as autosomal dominant or recessive disorder, with recessive DEB presenting more severe than dominant DEB. Skin cleavage in this disease subtype occurs below the lamina densa within the upper papillary dermis. Blisters appear from birth and with time are associated with atrophy and scarring of the skin and mucosa.1,8 In 100% of cases, mutations in the COL7A1 gene is the culprit of disease. With 11 clinical subtypes, recessive forms of DEB are highly associated with increased risk for developing squamous cell carcinoma.1,8 Metastatic squamous cell carcinoma is the leading cause of death in patients with recessive DEB with renal failure as a close second important cause of death among adults with this disease.10
Kindler epidermolysis bullosa (KEB)
KEB or Kindler Syndrome is a very rare subtype of EB that is only inherited in an autosomal recessive fashion, with only 250 cases reported worldwide.11 In this disease subtype, multiple skin cleavage planes are present in the intraepidermal, intralaminar lucida, and/or the sublamina densa. This relates to the genetic mutation in FERMT1 which encodes for an intracellular protein of focal adhesions in many skin layers.1 Characteristically, blisters in KEB are generalized at birth and progress to poikilodermatous pigmentation with associated photosensitivity.8 Similar to recessive DEB and JEB, there is also mucosal involvement.
Treatments
Prevention
The general principle for managing EB is supportive and monitoring. There are currently no approved treatments for EB and the primary management strategy is preventing the formation of blisters, proper wound care, and early recognition of secondary extracutaneous associations.12 In addition to preventative and supportive care, patients with EB should also undergo frequent laboratory and imaging tests to assess for development of sequela of disease.13
Symptom control
One of the common clinical symptoms of EB is pain and pruritus. Management of these clinical features is vital to improving a patient’s quality of life. General pain management regimens with a stepwise approach can be implemented depending on the severity of presentation.14 Additionally, antihistamines, topical steroids, and antidepressants are viable options for pruritic symptoms.14 Mucosal involvement is common across many forms of EB and compromises a patient’s nutritional status due to pain with feeding by mouth.8 For this reason, nutritional support is another vital component when managing EB. Age-appropriate supplementations and vitamins are a recommended part of this management strategy.15 There is an emphasis for increased protein consumption in this patient population due to the increased loss of protein with blistering and increased skin turnover.15 Extracutaneous complications of EB are often severe and require further management to prevent worsening of symptoms. Most notably, osteoporosis and squamous cell carcinoma are common sequela of EB that requires specialists to follow and manage the manifestations of these complications.
On the horizon
There is currently no approved treatment for EB, however, research related to the treatment options for EB are ongoing. Cutaneous gene therapy, cell-based therapies, protein replacement, and even read-through drug therapies are currently being evaluated as potential treatment options for EB.
Cutaneous gene therapy
Autologous transgenic keratinocytes transduced with a retroviral vector containing unmutated LAMB3 regenerated fully functioning epidermis of a seven-year-old with JEB after one month of treatment.16 This showcases promise in pursuit of cutaneous gene therapies for JEB and other subtypes of EB. A clinical trial held by Stanford University (NCT01263379) is investigating the use of genetically engineered autologous keratinocytes that have been transduced with a retroviral vector that contains the COL7A1 gene. Preliminary results of this study indicated wound healing to variable degrees in patients with recessive DEB, however the grafted sites had general decline over the course of one year.17 Currently, there is a phase 3 clinical trial sponsored by Abeona Therapeutics, Inc (NCT04227106) which continues to evaluate the safety and efficacy of use of this therapy. Recently in 2022, a randomized, placebo-controlled study, phase 1 and 2 clinical trial (NCT03536143), determined that topical Beremagene Geperpavec was easily administered, well tolerated, and promoted wound healing in patients with recessive DEB.18
Cell-based therapy
Two forms of cell-based therapies available include intradermal allogenic fibroblast injections or stem cell therapies. Three different studies utilized intradermal injections of fibroblasts in patients with recessive DEB and detected an increase in expression of type VII collagen,19,20 more rapid wound healing,20 and greater reduction in erosion of affected areas.21 Although effective, the results of these treatments ranged from only a few days to weeks. Allogeneic bone marrow transplantation allows for reprogramming of keratinocytes to produce non-defective proteins. In June 2022, allogenic skin-derived mesenchymal stem cells (allo-APZ2-EB) was granted orphan drug designation by the FDA for its immunomodulatory, anti-inflammatory, and tissue healing properties in the treatment of recessive DEB.22
Protein replacement therapy
Since type VII collagen is present in the skin in moderate amounts with a low turnover rate, direct application of the molecule could be a realistic approach to treating DEB. These theories have been tested in the animal models but require further investigation and research to fully understand the effects of protein therapies in the clinical setting.23
Read-through drug therapy
Gentamicin is a bactericidal broad-spectrum antibiotic that inhibits bacterial protein synthesis by binding to the 30S ribosome. A retrospective study considered the clinical effects of systemic gentamicin application in infants with severe generalized JEB and found that gentamicin had a positive impact on skin fragility and quality of life.24 Further, a clinical trial held by Oslo University Hospital (NCT04644627) investigated the effectiveness of topical gentamicin therapy in the treatment of EB. Another nonrandomized clinical trial studied IV gentamicin therapy and found restoration of laminin 332 functionality in patients with JEB.25 Gentamicin proves to be an important short-term therapy in treating EB and improving skin integrity.
Conclusion
Epidermolysis bullosa is the worst disease you have never heard of. There is currently no FDA approved treatment for EB and its many subtypes. The focus for EB specialists and researchers is heavily involved in research of new treatment modalities. Further investigation is warranted for this patient population given the detrimental effects of EB on a patient’s quality of life and life expectancy if complicated by chronic wound infections or sepsis.
References
1. Has C, Bauer JW, Bodemer C, et al. Consensus reclassification of inherited epidermolysis bullosa and other disorders with skin fragility. Br J Dermatol. Oct 2020;183(4):614-627. doi:10.1111/bjd.18921
2. Fine J-D. Epidemiology of Inherited Epidermolysis Bullosa Based on Incidence and Prevalence Estimates From the National Epidermolysis Bullosa Registry. JAMA Dermatology. 2016;152(11):1231-1238. doi:10.1001/jamadermatol.2016.2473
3. Understanding EB and its Classification. Accessed September 15, 2022. https://www.eb-researchnetwork.org/research/what-is-eb/
4. Fine JD, Johnson LB, Weiner M, Suchindran C. Cause-specific risks of childhood death in inherited epidermolysis bullosa. J Pediatr. Feb 2008;152(2):276-80. doi:10.1016/j.jpeds.2007.06.039
5. So JY TJ. Epidermolysis Bullosa Simplex. . In: Adam MP, Everman DB, Mirzaa GM, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle1998 Oct 7 [Updated 2022 Aug 4].
6. El Darouti MA, El Hawary MS, Abdel Hay RM. Kallin syndrome associated with vitiligo. Clin Exp Dermatol. Jan 2015;40(1):35-8. doi:10.1111/ced.12463
7. Ciubotaru D, Bergman R, Baty D, et al. Epidermolysis bullosa simplex in Israel: clinical and genetic features. Arch Dermatol. Apr 2003;139(4):498-505. doi:10.1001/archderm.139.4.498
8. Fine J-D. Inherited epidermolysis bullosa. Orphanet Journal of Rare Diseases. 2010;5 (12)doi:https://doi.org/10.1186/1750-1172-5-12
9. Fine J-D. Junctional Epidermolysis Bullosa (JEB, Junctional EB, EB Atrophicans). Dermatology Advisor2017.
10. Fine JD, Johnson LB, Weiner M, et al. Inherited epidermolysis bullosa and the risk of death from renal disease: experience of the National Epidermolysis Bullosa Registry. Am J Kidney Dis. Oct 2004;44(4):651-60.
11. MedlinePlus. Kindler Syndrome. Internet. National Library of Medicine (US). Updated June 01, 2016. Accessed September 25, 2022, https://medlineplus.gov/genetics/condition/kindler-syndrome/
12. Bruckner AL, Losow M, Wisk J, et al. The challenges of living with and managing epidermolysis bullosa: insights from patients and caregivers.
13. Martinez AE. Tests to monitor in patients with severe types of epidermolysis bullosa. Dermatol Clin. Apr 2010;28(2):271, ix. doi:10.1016/j.det.2010.01.005
14. Mellerio JE, Weiner M, Denyer JE, et al. Medical management of epidermolysis bullosa: Proceedings of the IInd International Symposium on Epidermolysis Bullosa, Santiago, Chile, 2005. Int J Dermatol. 2007:795-800. vol. 8.
15. Zidorio AP, Dutra ES, Leão DO, Costa IM. Nutritional aspects of children and adolescents with epidermolysis bullosa: literature review. An Bras Dermatol. Mar-Apr 2015;90(2):217-23. doi:10.1590/abd1806-4841.20153206
16. Hirsch T, Rothoeft T, Teig N, et al. Regeneration of the entire human epidermis using transgenic stem cells. Nature. Nov 16 2017;551(7680):327-332. doi:10.1038/nature24487
17. Siprashvili Z, Nguyen NT, Gorell ES, et al. Safety and Wound Outcomes Following Genetically Corrected Autologous Epidermal Grafts in Patients With Recessive Dystrophic Epidermolysis Bullosa. Jama. Nov 1 2016;316(17):1808-1817. doi:10.1001/jama.2016.15588
18. Gurevich I, Agarwal P, Zhang P, et al. In vivo topical gene therapy for recessive dystrophic epidermolysis bullosa: a phase 1 and 2 trial. Nat Med. Apr 2022;28(4):780-788. doi:10.1038/s41591-022-01737-y
19. Wong T, Gammon L, Liu L, et al. Potential of fibroblast cell therapy for recessive dystrophic epidermolysis bullosa. J Invest Dermatol. Sep 2008;128(9):2179-89. doi:10.1038/jid.2008.78
20. Venugopal SS, Yan W, Frew JW, et al. A phase II randomized vehicle-controlled trial of intradermal allogeneic fibroblasts for recessive dystrophic epidermolysis bullosa. J Am Acad Dermatol. Dec 2013;69(6):898-908.e7. doi:10.1016/j.jaad.2013.08.014
21. Petrof G, Martinez-Queipo M, Mellerio JE, Kemp P, McGrath JA. Fibroblast cell therapy enhances initial healing in recessive dystrophic epidermolysis bullosa wounds: results of a randomized, vehicle-controlled trial. Br J Dermatol. Nov 2013;169(5):1025-33. doi:10.1111/bjd.12599
22. Kiritsi D, Dieter K, Niebergall-Roth E, et al. Clinical trial of ABCB5+ mesenchymal stem cells for recessive dystrophic epidermolysis bullosa. JCI Insight. Nov 22 2021;6(22)doi:10.1172/jci.insight.151922
23. Remington J, Wang X, Hou Y, et al. Injection of recombinant human type VII collagen corrects the disease phenotype in a murine model of dystrophic epidermolysis bullosa. Mol Ther. Jan 2009;17(1):26-33. doi:10.1038/mt.2008.234
24. Hammersen J, Neuner A, Wild F, Schneider H. Attenuation of Severe Generalized Junctional Epidermolysis Bullosa by Systemic Treatment with Gentamicin. Dermatology. 2019;235(4):315-322. doi:10.1159/000499906
25. Mosallaei D, Hao M, Antaya RJ, et al. Molecular and Clinical Outcomes After Intravenous Gentamicin Treatment for Patients With Junctional Epidermolysis Bullosa Caused by Nonsense Variants. JAMA Dermatol. Apr 1 2022;158(4):366-374. doi:10.1001/jamadermatol.2021.5992
Articles in this issue
about 3 years ago
New Tech for Skinabout 3 years ago
Update on New Laser Treatments for Acneabout 3 years ago
Are You Prepared for Potential Tax Law Changes?about 3 years ago
Genetic Profiling and the Future of Therapeutics in Dermatologyabout 3 years ago
Low-Dose Oral Minoxidil for Hair Growthabout 3 years ago
The Role of Precision Medicine in the Management of Melanomaabout 3 years ago
The Latest Buzz in Atopic Dermatitisabout 3 years ago
Hand Healthabout 3 years ago
Hidradenitis Suppurativa: Current and Future TreatmentsNewsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.