- Dermatology Times, November 2022 (Vol. 43. No. 11)
- Volume 43
- Issue 11
Continuing Medical Education: Three Things to Know About Pustular Psoriasis
Pustular psoriasis (PP) is a rare and potentially serious inflammatory skin disorder affecting about 1% of patients with psoriasis.
Pustular psoriasis (PP) is a rare and potentially serious inflammatory skin disorder affecting about 1% of patients with psoriasis.1,2 Rapid diagnosis and fast-acting treatment is needed to properly manage this disease. Here are 3 things you should know about PP:
1. Autoinflammatory responses dominate pustular psoriasis
PP is typically idiopathic, and the immunopathogenic mechanisms linked to it are complex and indicate dysregulation of the innate immune response.3 Significant contributions from interleukins (ILs), tumor necrosis factor alpha (TNF-α) and interferons to the disease mechanism highlight the autoinflammatory nature of PP.4 The TNF-α/IL-23/IL-17/IL-22 axis and functional activation of the IL-36 receptor (IL-36R) are closely related processes in PP.5 Of these, the robust role of IL-1 and IL-36 cytokine family members have been drug targets of recent interest.4
IL-36 is a member of the IL-1 superfamily that interacts with IL-36R on various cell types in the skin and blood.6 Keratinocytes release IL-36 after activation of toll-like receptor 9 on dendritic cells, treatment with certain drugs, the presence of pathogen-associated molecular patterns and environmental factors. IL-36 subtypes α, β and γ bind to IL-36R to generate a proinflammatory signal via the nuclear factor kappa B and mitogen-activated protein (MAP) kinase signaling pathways.6 In healthy cells, skin homeostasis is maintained by IL-36 receptor antagonist (IL-36RA). Nearly a quarter of generalized pustular psoriasis (GPP) is linked to IL-36RA genetic deficiency.6 A cycle of inflammation ensues with T helper (Th)1 and Th17 effector cell differentiation and endothelial cell activation, among other effects.6 Mutations within IL36RN and other involved genes including CARD14, AP1S3 and IL1RN are implicated in PP.7-10 Illumination of this complex pathophysiology continues to expand options for targeted therapies.
2. Risk factor assessment facilitates diagnosis
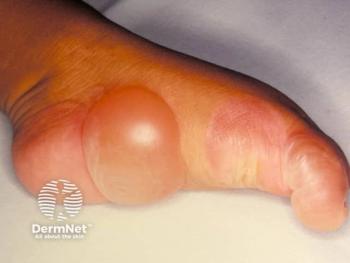
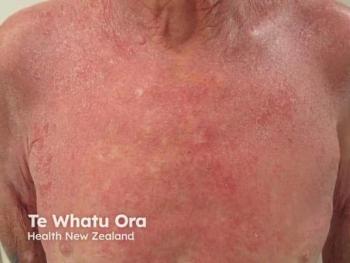
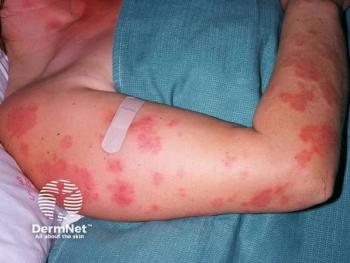
Two forms of adult-onset PP are generalized pustular psoriasis (GPP) and palmoplantar pustular psoriasis (PPP). Consensus definitions of GPP and PPP from the European Rare and Severe Psoriasis Expert Network have streamlined diagnosis and assessment. With GPP, sterile macroscopic pustules form on non-acral skin with or without systemic inflammation, with or without psoriasis vulgaris and with relapsing or persistent disease.11 A study with 102 patients identified the mean flare duration to be 10.3 days.2 Careful management is warranted to prevent life-threatening complications like sepsis and cardiorespiratory failure.3 PPP presents as sterile and macroscopic pustules on palms or soles with or without psoriasis vulgaris.11 PPP can be painful, disabling and persist for more than 3 months.12
Conditions mimicking GPP include drug hypersensitivity reactions (most common), miliaria, atopic dermatitis with secondary infection, subcorneal pustular dermatoses, acute generalized exanthematous pustulosis and tinea. Differential diagnoses for PPP include tinea and dyshidrotic eczema.13,14 Important laboratory tests for patients with suspected PP include complete blood counts, metabolic panels, erythrocyte sedimentation rate, C-reactive protein, proteinemia, calcium levels and blood/urine cultures.15 Histopathological diagnosis following biopsy can identify psoriasiform changes specific to PPP.16 Discomfort associated with biopsy for patients with PPP may outweigh its benefit in select cases.
Available assessment criteria, including the Generalized Pustular Psoriasis Physician Global Assessment (GPPGA), the Generalized Pustular Psoriasis Area and Severity Index (GPPASI) and Japanese Dermatological Association GPP severity criteria, have provided comprehensive clinical disease measures specifically for these patients.17 Risk factor-based assessment is critical to long-term management of PP. Withdrawal from systemic corticosteroids can trigger GPP flares.18 Family history, pregnancy and upper respiratory tract infections are also important indicators of risk.2 An analysis of 203 patients aimed at finding clinical and demographic factors relevant to PPP identified severe disease more frequently in women and current smokers than in men.12 Due to frequent recurrence, the threshold for testing should be lower in patients previously diagnosed with PP.
“Generalized pustular psoriasis is associated with systemic inflammation. We see patients present, and they are diffusely red, their legs are swollen, their cardiac output is very high, they're having a lot of pain, a lot of inflammation, and they are really sick.” —Alan Menter, MD
3. Emerging therapies offer targeted treatment options
Traditional first-line options for GPP include systemic acitretin, cyclosporine and methotrexate.19 For severe disease, biologics like infliximab, IL-17 inhibitors (secukinumab, ixekizumab and brodalumab) or IL-23 inhibitors (ustekinumab and guselkumab) can be used, sometimes in combination with another nonbiologic agent.19 Current biologics, however, can have access limitations and have primarily been tested for use in plaque psoriasis, which may not translate to PP. Fast-acting and powerful treatments with sustained efficacy are still needed.3
Emerging treatments address underlying causes of disease, including investigational IL-36 inhibition therapy. Spesolimab is an anti–IL-36R monoclonal antibody designed to treat GPP. In the phase 2 Effisayil 1 trial (NCT038782792), 53 patients received a 900-mg intravenous (IV) dose of spesolimab versus placebo.20 After 1 week, 19 of 35 treated patients had a GPPGA subscore of 0, compared with 1 of 18 patients in the placebo group (P = .02). However, treatment was also associated with infections (47% of patients given spesolimab) and systemic drug reactions (46% of patients given spesolimab).20
Phase 2 trials are currently underway for PPP. Future studies will also be needed to determine the relapse rate following treatment with spesolimab. Imsidolimab is another antibody inhibitor of IL-36R intended for treatment of GPP. In the single arm, open-label phase 2 GALLOP trial (NCT03619902), patients received a 750-mg IV dose on day 1, followed by monthly 100-mg subcutaneous doses for 16 weeks.21 Six of 8 patients achieved the primary end point response on the Clinical Global Impression (CGI) Scale. Two patients dropped out early, including one who contracted a Staphylococcus aureus infection.21 Phase 3 trials for GPP are currently ongoing. The addition of anti–IL-36 pathway- and similar targeted agents to the therapeutic arsenal may reshape the future of PP management.
Key References
3. Menter A, Van Voorhees AS, Hsu S. Pustular psoriasis: a narrative review of recent developments in pathophysiology and therapeutic options. Dermatol Ther (Heidelb). 2021;11(6):1917-1929. doi:10.1007/s13555-021-00612-x
4. Johnston A, Xing X, Wolterink L, et al. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J Allergy Clin Immunol. 2017;140(1):109-120. doi:10.1016/j.jaci.2016.08.056
20. Bachelez H, Choon SE, Marrakchi S, et al. Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385(26):2431-2440. doi:10.1056/NEJMoa2111563
For a full list of references go to www.gotoper.com/vcs22psp-art
This activity was written by PER® editorial staff under faculty guidance and review.
Faculty, Staff and Planners’ Disclosures
In accordance with ACCME Guidelines, PER® has identified and resolved all COI for faculty, staff and planners prior to the start of this activity by using a multistep process.
Disclosures (Dr Menter): Grant/Research Support: Abbott Laboratories, Amgen, Argenx, BV, Boehringer Ingelheim, Novartis, Pfizer, Sun Pharma, UCB. Consultant: Abbott Laboratories, Amgen, Biocon, Eli Lilly and Company, Janssen Biotech, Leo Pharma, Mindera Health, Novartis, Sun Pharma, UCB. Speaker’s Bureau: Abbott Laboratories, Amgen, Eli Lilly and Company, Sun Pharma, UCB.
The staff of Physicians’ Education Resource®, LLC have no relevant financial relationships with ineligible companies.
To learn more about this topic, including information on diagnosing and treating pustular psoriasis, go to www.gotoper.com/vcs22psp-act
Release Date: June 1, 2022
Expiration Date: June 1, 2023
Learning Objectives
Upon successful completion of this activity, you should be better prepared to:
- Identify the clinical manifestations of pustular psoriasis
- Apply evidence-based strategies to support a prompt and timely diagnosis Describe inflammatory mediators that are implicated in the pathophysiology of pustular psoriasis
- Assess clinical trial evidence evaluating investigational therapies for the management of pustular psoriasis
Accreditation/Credit Designation
Physicians’ Education Resource®, LLC, is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians.
Physicians’ Education Resource®, LLC, designates this enduring material for a maximum of 0.25 AMA PRA Category 1 Credits™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Acknowledgment of Commercial Support
This activity is supported by an educational grant from Boehringer Ingelheim Pharmaceuticals, Inc.
Off-Label Disclosure/Disclaimer
This activity may or may not discuss investigational, unapproved or off-label use of drugs. Learners are advised to consult prescribing information for any products discussed. The information provided in this activity is for accredited continuing education purposes only and is not meant to substitute for the independent clinical judgment of a health care professional relative to diagnostic, treatment or management options for a specific patient’s medical condition. The opinions expressed in the content are solely those of the individual faculty members, and do not reflect those of PER® or any company that provided commercial support for this activity.
Instructions for Participation/ How to Receive Credit
1. Read this activity in its entirety.
2. Go to www.gotoper.com/vcs22psp-art to access and complete the post-test.
3. Answer the evaluation questions.
4. Request credit using the drop-down menu. You may immediately download your certificate.
Articles in this issue
about 3 years ago
New Tech for Skinabout 3 years ago
Update on New Laser Treatments for Acneabout 3 years ago
Are You Prepared for Potential Tax Law Changes?about 3 years ago
Genetic Profiling and the Future of Therapeutics in Dermatologyabout 3 years ago
Low-Dose Oral Minoxidil for Hair Growthabout 3 years ago
The Role of Precision Medicine in the Management of Melanomaabout 3 years ago
The Latest Buzz in Atopic Dermatitisabout 3 years ago
Hand Healthabout 3 years ago
Hidradenitis Suppurativa: Current and Future Treatmentsabout 3 years ago
How to Have a Proper Presence on Social MediaNewsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.