- Dermatology Times, July 2022 (Vol. 43. No. 7)
- Volume 43
- Issue 7
Alopecia Areata Gets FDA-Approved Drug
Approval of a novel oral JAK inhibitor specifically indicated for this challenging condition fills a long unmet need.
Alopecia areata (AA) is a historically challenging-to-treat condition, with few effective therapy options. In June 2022, the FDA ended the wait for a specific treatment for this condition with its approval of the novel oral J Kinase (JAK) inhibitor baricitinib (Olumiant; Eli Lilly and Company and Incyte) as the first systemic treatment specifically indicated for severe AA.1
“Access to safe and effective treatment options is crucial for the significant number of Americans affected by severe alopecia,” said Kendall Marcus, MD, director of the Division of Dermatology and Dentistry in the FDA’s Center for Drug Evaluation and Research, Washington, DC, in the FDA announcement. “This approval will help fulfill a significant unmet need for patients with severe alopecia areata.”
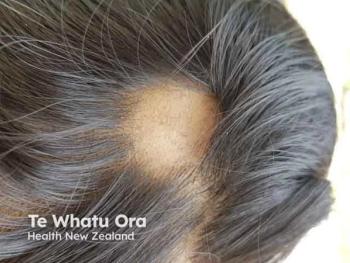
AA, which affects approximately 6.8 million people in the United States,2is a common autoimmune disorder with a still-unknown etiology typically resulting in unpredictable patchy hair loss on the body. The clinical presentations for AA can vary from 1 to multiple well-defined patches to more diffuse or total hair loss on the scalp (alopecia totalis) or hair loss on the entire body (alopecia universalis).
This can result in significant anxiety and depression and can have a devastatingly negative psychosocial impact, particularly when symptoms are severe—further impacting a patient’s quality of life.3
“Unfortunately, AA is sometimes wrongfully characterized as a cosmetic disease,” said Lotus Mallbris, MD, PhD, vice president of global immunology development and medical affairs at Eli Lilly and Company in Chicago, Illinois.
“These patients not only have patchy hair loss but can also lose their eyebrows and eyelashes, and when this happens, they cannot recognize themselves in the mirror anymore. This can be deeply traumatic for patients, and they deserve a safe and effective treatment for their disease. Baricitinib is a huge therapeutic breakthrough for this patient population.
”The data behind the historic FDA approval for severe AA comes from 2 trials4, BRAVE-AA1 (NCT03570749)5 and BRAVE-AA2 (NCT03899259)6, which were randomized, double-blind, placebo-controlled studies including patients presenting with at least 50% scalp hair loss as measured by the Severity of Alopecia Tool for more than 6 months.
Study participants were placed into 1 of 3 arms and received placebo, 2 mg of baricitinib, or 4 mg of baricitinib every day. The primary end point of the trials was the proportion of patients who achieved at least 80% scalp hair coverage at week 36.
Results from BRAVE-AA1 showed that 22% of the 184 patients who received 2 mg of baricitinib and 35% of the 281 patients who received 4 mg of baricitinib achieved adequate scalp hair coverage compared with 5% of the 189 patients who received a placebo. In BRAVE-AA2, 17% of the 156 patients who received 2 mg of baricitinib and 32% of the 234 patients who received 4 mg of baricitinib achieved adequate scalp hair coverage vs 3% of the 156 patients who received a placebo.
“We can freely say that 1 in 5 adults with a 2-mg daily dose of baricitinib can achieve 80% of regrowth of hair coverage,” Mallbris said. “Similarly, 1 in 3 adults can achieve 80% coverage with a 4-mg daily dose of baricitinib. This is the first time that patients with severe AA will get an FDA-approved oral systemic agent demonstrating excellent efficacy in the treatment and management of their disease.
”Clinicians can titer up or down based on the patient’s hair loss severity and individual needs. Once an adequate response is achieved, the daily dose can be reduced to 2 mg, giving clinicians and their patients flexibility in disease management. AA is mediated by cytokines interferon-γ and IL-15, which signal through the JAK-STAT pathway. Baricitinib, a JAK inhibitor, modulates the activity of these 2 cytokines, leading to hair regrowth in AA.4
“The approval of baricitinib for severe AA means that patients with this awful disease may now finally get treatment,” said Brett King, MD, PhD, associate professor of dermatology at Yale School of Medicine in New Haven, Connecticut. “There has been nothing for these patients that has adequately addressed their severe AA symptoms, and baricitinib looks to be a game changer in the treatment and management of patients suffering from this psychologically devastating disease.
”Different treatment options, both local and systemic, have been used for patients with severe AA, but none have been proven effective long term. According to King, the JAK inhibitor tofacitinib (Xeljanz; Pfizer Inc) used off label has shown some efficacy in severe cases, but the dawn of baricitinib heralds a new era in the treatment and management of patients with severe AA.
Common adverse events (AEs) associated with baricitinib, as with other small molecule inhibitors, include upper respiratory tract infections, headache, acne, hyperlipidemia, increased creatine phosphokinase levels, urinary tract infection, liver enzyme elevations, folliculitis, fatigue, lower respiratory tract infections, nausea, genital yeast infections, anemia, neutropenia, abdominal pain, herpes zoster, and weight increase.
The AEs that occurred in the 2 BRAVE clinical trials were all mild and manageable, and according to King, many of these AEs and others that may occur with JAK inhibitors are similar to those seen with other systemic immunomodulatory or immunosuppressive therapies.
Baricitinib comes with a boxed warning for serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis1 but still received priority review and breakthrough therapy designations for this indication. Speaking to the boxed warning, King said he has prescribed JAK inhibitors off label—mostly tofacitinib—to hundreds of his adult and adolescent patients over the past 8 years, primarily for AA but also for atopic dermatitis and other skin diseases.
“I have always spent a lot of time explaining to patients/families the safety data and evolving safety data so that they are able to make an informed decision,” King said. “Although some ask for details, others just want my impression of the potential risks involved. After meticulous review and [being] equipped with information...to weigh potential risks against potential benefits, patients make their informed choice. It is a true shared treatment and management decision-making process.”
Because hair regrowth in patients with severe AA can take time, King suggested adding oral minoxidil to the baricitinib treatment regimen to help increase both the speed of hair regrowth and the number of patients who respond to treatment.
“This is a very exciting time for AA patients, and there is nothing like growing hair for somebody with severe AA,” King said. “It is truly life changing for patients and incredibly rewarding to make happen.”
Disclosures
King has received honoraria and/or consulting fees from Aclaris Therapeutics Inc, Almirall, Arena Pharmaceuticals, Bristol Myers Squibb, Concert Pharmaceuticals Inc, Dermavant Sciences, Eli Lilly and Company, Pfizer Inc, TWi Biotechnology Inc, and Viela Bio Inc. He has been a clinical trial investigator for Arena Pharmaceuticals, Concert Pharmaceuticals Inc, Eli Lilly and Company, National Institutes of Health, and Pfizer Inc. He is on speaker bureaus for Pfizer Inc, Regeneron, and Sanofi Genzyme.
References
- FDA approves first systemic treatment for alopecia areata. News release. FDA. June 13, 2022. Accessed June 13, 2022. https://www.fda.gov/news-events/press-announcements/fda-approves-first-systemic-treatment-alopecia-areata
- What you need to know about alopecia areata. National Alopecia Areata Foundation. Accessed June 15, 2022. https://www.naaf.org/alopecia-areata
- Mesinkovska N, King B, Mirmirani P, Ko J, Cassella J. Burden of illness in alopecia areata: a cross-sectional online survey study. J Investig Dermatol Symp Proc. 2020;20(1):S62-S68. doi:10.1016/j.jisp.2020.05.007
- King B, Ohyama M, Kwon O, et al; BRAVE-AA Investigators. Two phase 3 trials of baricitinib for alopecia areata. New Engl J Med.2022;386(18):1687-1699. doi:10.1056/NEJMoa21103435.
- A study of baricitinib (ly3009104) in participants with severe or very severe alopecia areata (BRAVE-AA1). Updated February 3, 2022. Accessed June 17, 2022. https://clinicaltrials.gov/ct2/show/NCT03570749
- A study of baricitinib (ly3009104) in adults with severe or very severe alopecia areata (brave-AA2). Updated January 26, 2022. Accessed June 17, 2022. https://www.clinicaltrials.gov/ct2/show/NCT03899259
Articles in this issue
over 3 years ago
Optimize Acids, Peels in Acne Scar Treatmentover 3 years ago
Trends in Clean, Sustainable, and Green Skin Care, Cosmeticsover 3 years ago
The Inside Look on the Dupilumab Approvalover 3 years ago
Factors Affecting Patients Adherence in Topical Therapiesover 3 years ago
Innovation in Psoriasis TherapiesNewsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.