
New findings at Elevate Derm highlight remibrutinib's rapid and sustained efficacy in reducing chronic spontaneous urticaria symptoms, offering hope for patients.

New findings at Elevate Derm highlight remibrutinib's rapid and sustained efficacy in reducing chronic spontaneous urticaria symptoms, offering hope for patients.
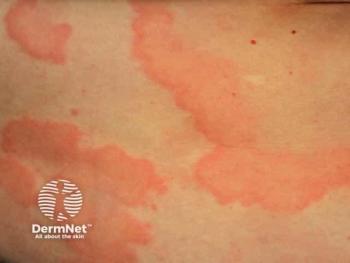
Explore how genetic factors influence acute and chronic urticaria, revealing potential for personalized treatment strategies.

Jasper Therapeutics reveals promising results from briquilimab trials for chronic spontaneous urticaria, showcasing strong efficacy and safety despite manufacturing challenges.

Researchers found CU was associated with increased odds of developing anxiety, depression, PTSD, and substance use disorder, among other conditions.
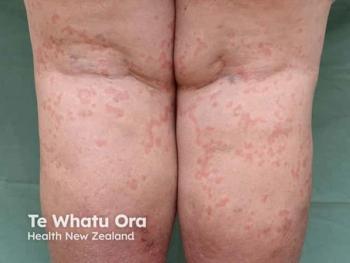
Treatment with rupatadine led to improvements in CSU and chronic inducible urticaria.

The monoclonal anti-KIT antibody exhibited promise in adults with moderate to severe antihistamine-refractory CSU.

Research reveals a significant link between TNF-alpha serum levels and the severity of chronic spontaneous urticaria, suggesting new therapeutic avenues for treatment.

Put your dermatology expertise to the test with Dermatology Times’ interactive quiz series!

Recludix Pharma unveils a groundbreaking BTK SH2 inhibitor, showcasing superior selectivity and efficacy for chronic spontaneous urticaria treatment at SID 2025.

Dupilumab becomes the first targeted therapy approved for chronic spontaneous urticaria in more than a decade.

Having multiple therapeutic options would allow clinicians to tailor treatment and offer hope to patients who don't respond initially.

At AAAAI/WAO 2025, Bernstein explored new findings on rilzabrutinib and dupilumab in CSU, highlighting treatment gaps and future therapies.

Phase 3 trial results presented at AAAAI/WAO 2025 highlight dupilumab's efficacy in reducing itch severity and urticaria activity in CSU patients.

Hawkes discusses urticaria subtypes, treatments, and emerging therapies like dupilumab and remibrutinib.

According to a phase 3 study, the omalizumab biosimilar saw comparable results with a good safety profile.