Acne cream seeks marketing authorizations in EU
Trifarotene may become the European Union’s first new retinoid molecule acne treatment for the face and trunk in 25 years following upcoming individual member state marketing approvals in 2020.
Trifarotene 50 mcg/g cream (AKLIEF, Galderma) may become the European Union’s first retinoid molecule treatment of acne in 25 years, according to a company release detailing the recent approval of the package leaflet, product characteristics and labelling in the European Decentralized Procedure by 16 member states.
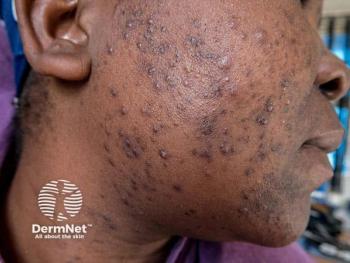
Trifarotene is a topical treatment for acne vulgaris in individuals 12 years or older when many papules, pustules and comedones are present on the face and trunk. It is currently the only topical retinoid that targets the most common retinoic acid receptor in the skin - RAR-y.
Professor Jerry Tan, M.D., University of Western Ontario, Windsor, ON, Canada, says investigations into new treatments for truncal acne are vital due to the condition affecting nearly half of individuals that have facial acne. He adds the condition frequently goes underdiagnosed and under-reported, which means that little scientific evidence regarding the management of truncal acne exists.
This news comes after results from two randomized phase 3 clinical studies (PERFECT 1 and 2) displayed low systematic exposure of trifarotene 50 mcg/g due to its quick metabolization in the liver with a half-life of five minutes.
Additionally, results from the studies showed the drug reduced inflammatory lesions in two weeks on the face and four weeks on the truncal areas compared to the control. Drug-related adverse mild-to-moderate reactions included scaling, erythema, dryness and burning on the application site.
“Acne can severely impact the quality of life and psychological well-being of those who suffer from it. The approval of AKLIEF will provide patients across Europe with a next-generation retinoid cream that has been shown to be fast and effective at treating acne on both the face and trunk,” states Thibaud Portal, Ph.D., global vice president of prescription medicines at Galderma.
The cream was also recently approved by the U.S. Food and Drug Administration in October 2019 and Health Canada in November 2019.
The next step is for each of the 16 member states to individually distribute marketing authorizations for AKLIEF in 2020.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.