- Dermatology Times, October 2020 (Vol. 41, No. 10)
- Volume 41
- Issue 10
My experience with surface radiotherapy
Superficial radiation offers a newer, FDA-approved office-based option that is safe. Steven A. Davis, M.D., shares his experience using surface radiotherapy to treat his patients with non-melanoma skin cancer.
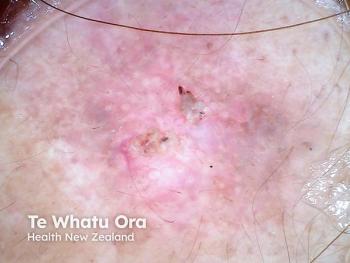
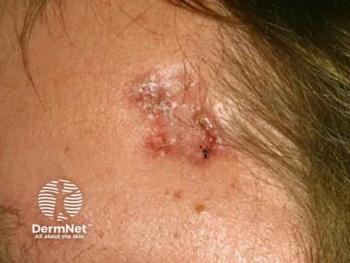
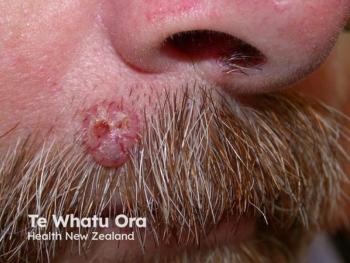
Recent advancements have brought X-rays safely into the dermatology clinic. This past year, I have incorporated surface radiotherapy (SRT), which is approved by the U.S. Food and Drug Administration (FDA), as a treatment option for select patients with non-melanoma skin cancer (NMSC).
I have treated more than 25,000 skin cancers surgically, which is the standard of care for the vast majority of patients with NMSC. But surgery is not ideal for every patient. Patients taking prescription blood thinners or those who cannot care for surgical wounds, have a history of delayed or insufficient wound healing, an aversion to surgery, or who wish to avoid potentially deforming surgery on areas like the ears and nose may be candidates for nonsurgical options. These considerations, plus the patient’s access to care, age and family support all factor into the evaluation of surgery versus superficial radiation treatment in the dermatology clinic. It’s a classic benefit: risk analysis.
In my patient population of largely Medicare-age skin cancer patients, there is generally higher risk for stress from prolonged procedures, post-operative bleeding, delayed wound healing and inability to carefully for treatment sites. During the COVID-19 threat, the risk from pro- longed face-to-face contact with the surgical team is an additional safety factor to consider.
A 0.5 cm basal cell carcinoma (BCC) on the posterior lower leg of a healthy 60-year-old patient is typically a better candidate for electrodesiccation and curettage or excision than the 80-year- old patient with diabetes who has a BCC on the pre-tibial surface. A Mohs procedure closed with a flap on the nasal sidewall may be excellent for a 70-year-old patient in good health, but SRT is a good option for a patient the same age who has a cardiac condition and is on blood thinners.
In contrast to the largely unregulated office- based “X-ray” treatment machines popular up into the 1960’s, today’s SRT devices are calibrated to precisely deliver electronically generated — in the 50 to 150 kilovolt (kv) range — photons directly to the skin surface, targeting the tumor area while sparing the surrounding healthy skin. Patients typically receive three treatments per week for a total of 15 to 18 treatments.
Electronic brachytherapy (eBt) offers a similar treatment option using slightly less X-ray energy and a sharper field edge that enables fewer treatments — eight to 12 — delivered twice weekly. Actual treatment time-on-tissue for both modalities averages one to two minutes per session (“fraction”), and the patient can be in and out of the office in less than 15 minutes. Both are approved by the FDA for treating NMSCs and have cure rates in the approximate range of 95+ %
In my experience, many patients don’t think about radiation as a treatment for skin cancer. When we inform patients that nonsurgical SRT can be delivered in our office with far fewer treatments, they can weigh its benefits and risks against other options. In my practice, these options have included simple and advanced surgery, imiquimod and injectable 5 fluorouracil. After favorable reviews of office-based SRT from some of my col- leagues, I decided to add it to my practice.
I chose the RADiant system from XStrahl, an international company that makes both superficial and orthovoltage radiation treatment devices. RADiant appealed to me because it provides both eBt and SRT modes in a single machine with a small footprint. This system does not use radioisotopes, the treatment room requires minimal shielding and the intuitive software interface holds treatment plans that are customized to each patient; thus, reducing the chance for human error.
We have now used our RADiant system for a year. There’s uniformly good patient satisfaction; treatments are brief and painless; most treatment effects, such as redness and scaling, are transient and well tolerated; and the treated sites heal with little to no scarring. While generally an attractive alternative to surgery, SRT/eBt has to be used judiciously to merit insurance coverage; while, at the same time, being cost-effective for the practitioner. Most devices on the market cost about $150K -$250K, and there are set-up fees and maintenance plans to consider.
Medicare with supplements typically covers SRT/eBt treatments. With Medicare Advantage and fully private plans, it’s wise to check with the carrier for coverage or need for pre-authorization. In terms of state regulatory requirements, SRT/ eBt are well within the domain of the dermatologist, though some states place different regulatory requirements on use of eBt. If you are considering adding SRT/eBt to your practice be sure you go over your state requirements with the company from whom you are buying your device.
Given my own practice’s volume of cases and patient mix, the RADiant system’s dual mode, ease of use and reasonable start-up costs have efficiently expanded the options I can offer patients to treat their NMSCs. For patients who are apprehensive or fearful of pain or surgical complications — and otherwise meet the indications for SRT/eBt, my RADiant system has become a welcome, less stressful alternative to traditional surgery.
Disclosure:
Dr. Steven Davis purchased his RADiant System and is not compensated for articles and presentations related to it.
Articles in this issue
over 5 years ago
Stem cell media boosts microneedling benefitsover 5 years ago
Cosmetic procedure complications in darker skinover 5 years ago
PDL, botulinumtoxinA improves rosacea symptomsover 5 years ago
Trial compares NMSC destructive techniquesover 5 years ago
Phenotype-based approach advances managementover 5 years ago
The retinoid revolutionover 5 years ago
Managing pandemic patient volumesover 5 years ago
I warned my patient – was it enough?over 5 years ago
Dupilumab outlasts cyclosporineNewsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.