- Dermatology Times, December 2019 (Vol. 40, No. 12)
- Volume 40
- Issue 12
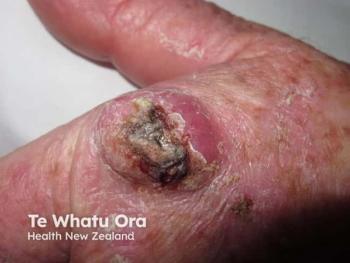
Genetic expression testing still a work in progress for melanoma
One expert believes that clinicians may want to seek a second pathologic opinion for particularly di fficult-to-diagnose melanocytic lesions before solely relying on the results of existing molecular assays.
Gene expression profiles used to aid in
“Existing commercial molecular tests for melanoma diagnosis can be useful for common subtypes but it’s important to recognize that they haven’t been completely validated and may not be helpful in some of the more challenging melanomas such as Spitzoid, nevoid, acral and mucosal melanoma,” says Jason C. Sluzevich, M.D., assistant professor of dermatology, department of dermatology, Mayo School of Medicine, Jacksonville, Fla., who recently spoke at the 4th Annual Mayo Clinic Cutaneous Oncology Symposium in Orlando.
Commercially available tests, such as the
At the time when the myPath test was created, Dr. Sluzevich says that it was designed using sets of melanomas that all experts agreed were melanoma, and it is from these samples that the genes of interest that were thought to predict whether something was benign or malignant were derived.
“It is important to know the limitations of the myPath Melanoma test, and to realize that because ambiguous melanocytic proliferations were not included in the defining data set, the test may not always be helpful in sorting out these challenging lesions,” he says.
According to Dr. Sluzevich, it may be better to get a second expert opinion rather than initially opting for the myPath Melanoma test especially for ambiguous Spitzoid proliferations and pigmented lesions on acral and mucosal sites.
The myPath Melanoma test also had lower performance characteristics in classifying desmoplastic melanoma as malignant, Dr. Sluzevich says, and was less sensitive and specific in diagnosing Spitzoid melanoma when compared to a FISH (Fluorescence in situ Hybridization) based method in published reports.
According to Dr. Sluzevich, it is important for clinicians to know the limitations of the myPath Melanoma test so they can properly evaluate a pathologic opinion.
“The bottom line is GEP-based testing for melanoma diagnosis is still a work in progress. Additional validation studies and the addition of other candidate genes in newer assays, perhaps specific to melanoma histologic subtype and anatomic site, may be seen in the future,” Dr. Sluzevich says.
Articles in this issue
about 6 years ago
Diet may impact squamous cell carcinoma riskabout 6 years ago
New resource for teens with atopic dermatitisabout 6 years ago
When is the right time to retire?about 6 years ago
40 years of champions in dermatologyabout 6 years ago
A novel target for rosacea treatmentabout 6 years ago
Should dermatologists embrace AI?about 6 years ago
Spironolactone efficacy may be similar to oral antibiotic Txabout 6 years ago
Hair growth treatmentsNewsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.