Dupilumab Improves Chronic Spontaneous Urticaria Symptoms
A recent statement by Regeneron noted that chronic spontaneous urticaria is the fifth disease associated with positive Phase 3 results involving the monoclonal antibody.
Phase 3 trials for the monoclonal antibody dupilumab (Dupixent; Sanofi and Regeneron) found that the drug significantly reduced itch and hives for patients with moderate-to-severe chronic spontaneous urticaria (CSU) compared to antihistamines used in the first trial of the LIBERTY-CUPID clinical program.
The Phase 3 trial, supported by Sanofi and Regeneron Pharmaceuticals, was the first trial to show how dupilumab effectively targeted IL-4 and IL-13 proteins in patients with chronic spontaneous urticaria.
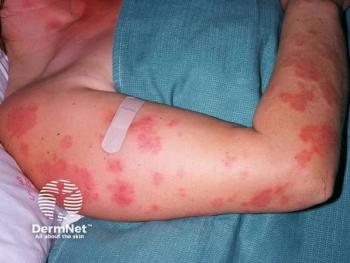
Chronic spontaneous urticaria is a chronic inflammatory disease associated with hives and swelling of the skin. While traditionally treated with antihistamines, the disease remained uncontrolled for up to 50% of patients due to limited available treatment options.
However, the 24-week treatment period in the recent Phase 3 trial of dupilumab showed a 63% reduction in itch severity and a 65% reduction in urticaria activity severity in patients with chronic spontaneous urticaria, which was roughly double the effectiveness of the placebo groups.
Chronic spontaneous urticaria was the fifth diseases for which dupilumab had positive Phase 3 data. Other diseases treated with dupilumab included atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyposis. It is also under investigation in eosinophilic esophagitis.
Researchers will continue with Study B of the LIBERTY-CUPID clinical program, which will study dupilumab in adults and adolescents who are symptomatic despite standard-of-care treatment and are intolerant or had incomplete responses to omalizumab.
“By early 2022, we look forward to reporting results from a second trial in patients who were unable to control their chronic spontaneous urticaria with another biologic medicine, as well as other trial results in additional dermatologic diseases,” George D. Yancopoulos, MD, PhD, President and Chief Scientific Officer at Regeneron said.
This article was originally published by our sister publication
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.