- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
Article
Breakthrough therapy designation granted to nemolizumab for pruritus
Author(s):
The United States Food and Drug Administration has granted Galderma’s nemolizumab a Breakthrough Therapy Designation following phase 2 results which suggests the drug is a considerable alleviant for pruritus associated with prurigo nodularis.
Significant developments are on track for patients with pruritus associated with prurigo nodularis after a recent announcement by the United State Food and Drug Administration.
RELATED: Atopic dermatitis drugs in development
Following an October presentation of phase 2 results at the 28th Annual Congress of the European Academy of Dermatology and Venereology in Madrid, the U.S. FDA has now granted Galderma a Breakthrough Therapy Designation for their pruritus treatment drug nemolizumab.1 The U.S. FDA grants up-and-coming therapies a Breakthrough Therapy Designation when a new drug or device has shown significantly improved treatment over the currently available options for serious or life-threatening conditions.
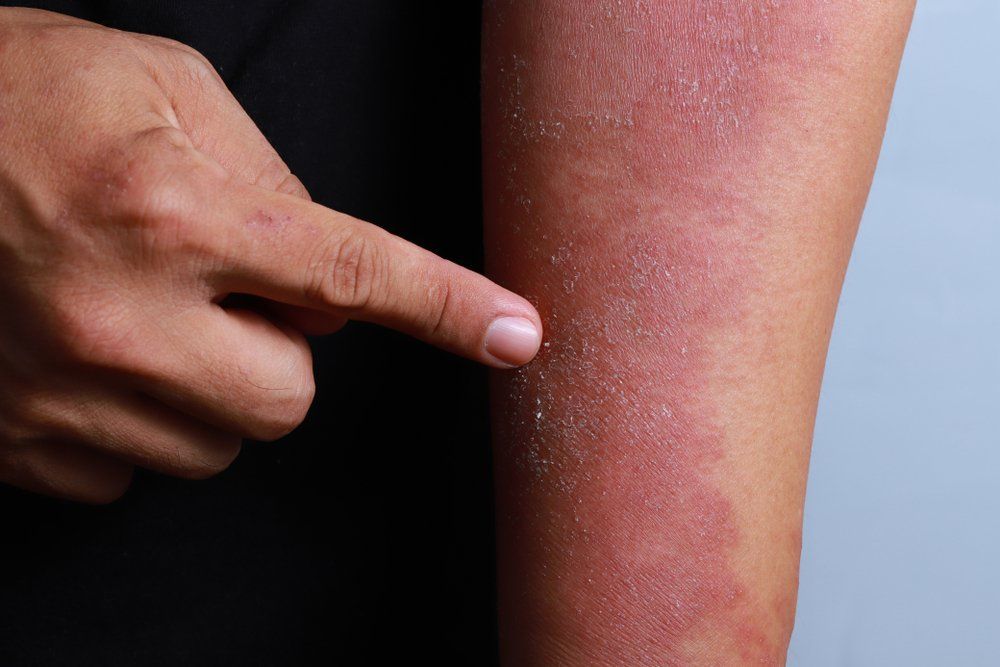
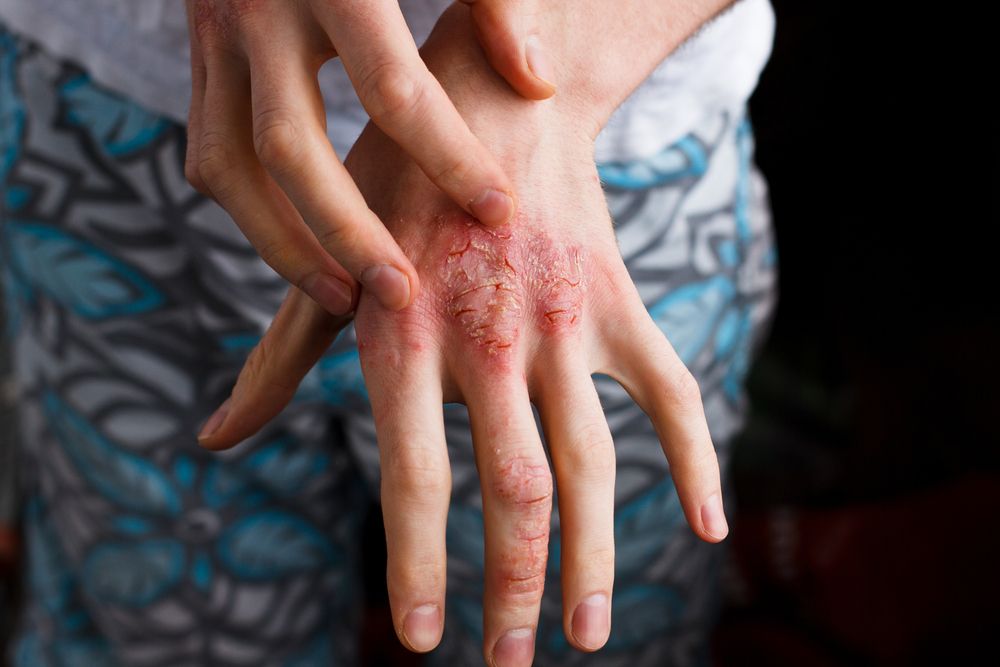
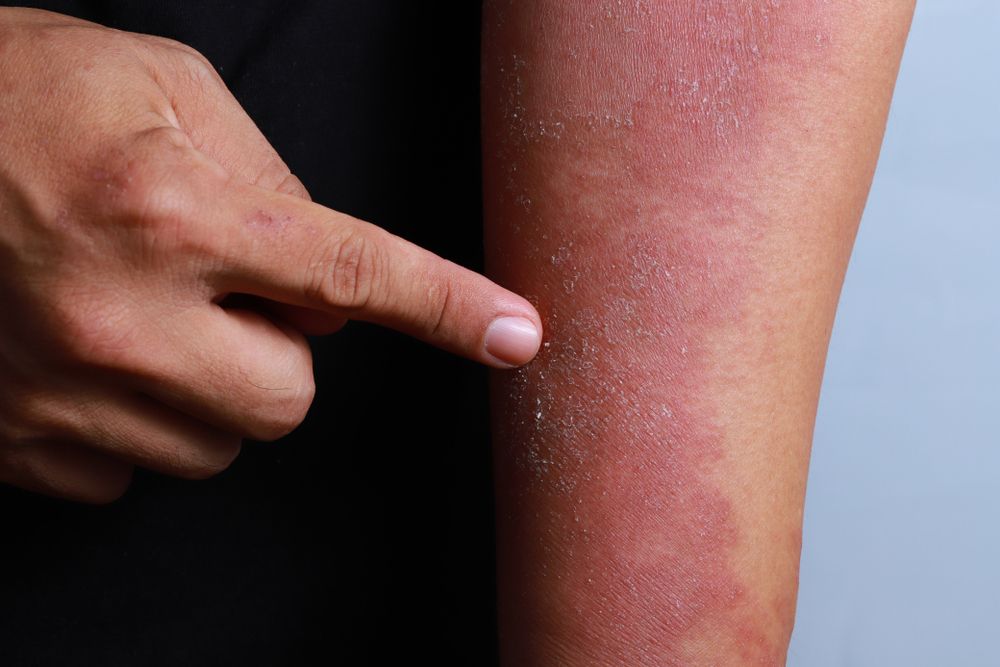
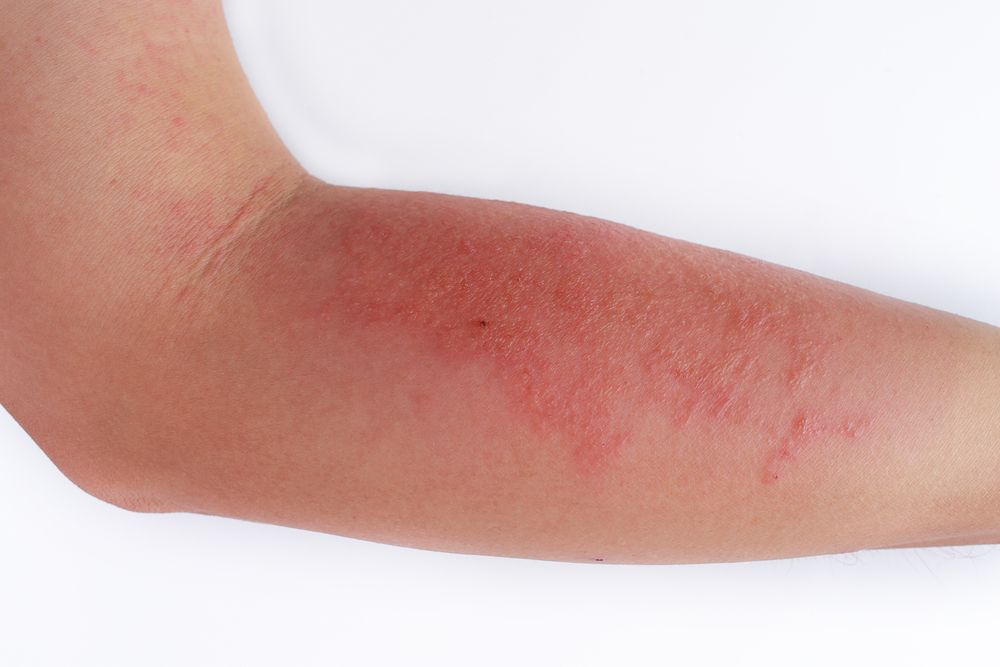
Nemolizumab is an investigational monoclonal antibody that blocks signaling to interleukin-31 (IL-31), which is the pathogenesis of pruritus and an exacerbator for atopic dermatitis.2 Pruritus is typically associated with prurigo nodularis, a persistent skin condition consisting of thick skin nodules that can cause severe itching.
Results of the phase 2 nemolizumab study involving moderate-to-severe prurigo nodularis patients showcased the drug’s considerably efficacy in improving skin lesions, measured by the Investigator Global Assessment (IGA), while simultaneously meeting the primary endpoint of a greater improvement in peak pruritus Numerical Rating Scale (PP NRS) from the baseline in comparison to the placebo.
“We are very excited by the prospect of offering a treatment option to doctors, and their patients with prurigo nodularis, where currently there are no registered therapy options. We are fully committed to continue driving forward the overall nemolizumab development program,” says Dr. Thibaud Portal, Galderma Global Vice President of Prescription medicines.
READ MORE: New biologics target a number of dermatoses
Beginning in 2020, Galderma will launch phase 3 clinical trials with nemolizumab in adult patients with prurigo nodularis. The company has previously announced that they have enrolled the first patients of phase 3 involving nemolizumab and adult patients with moderate-to-severe atopic dermatitis.
While nemolizumab shows significant promise for pruritus and atopic dermatitis, the drug is still under clinical review and has yet to be fully evaluated by any regulatory authority.