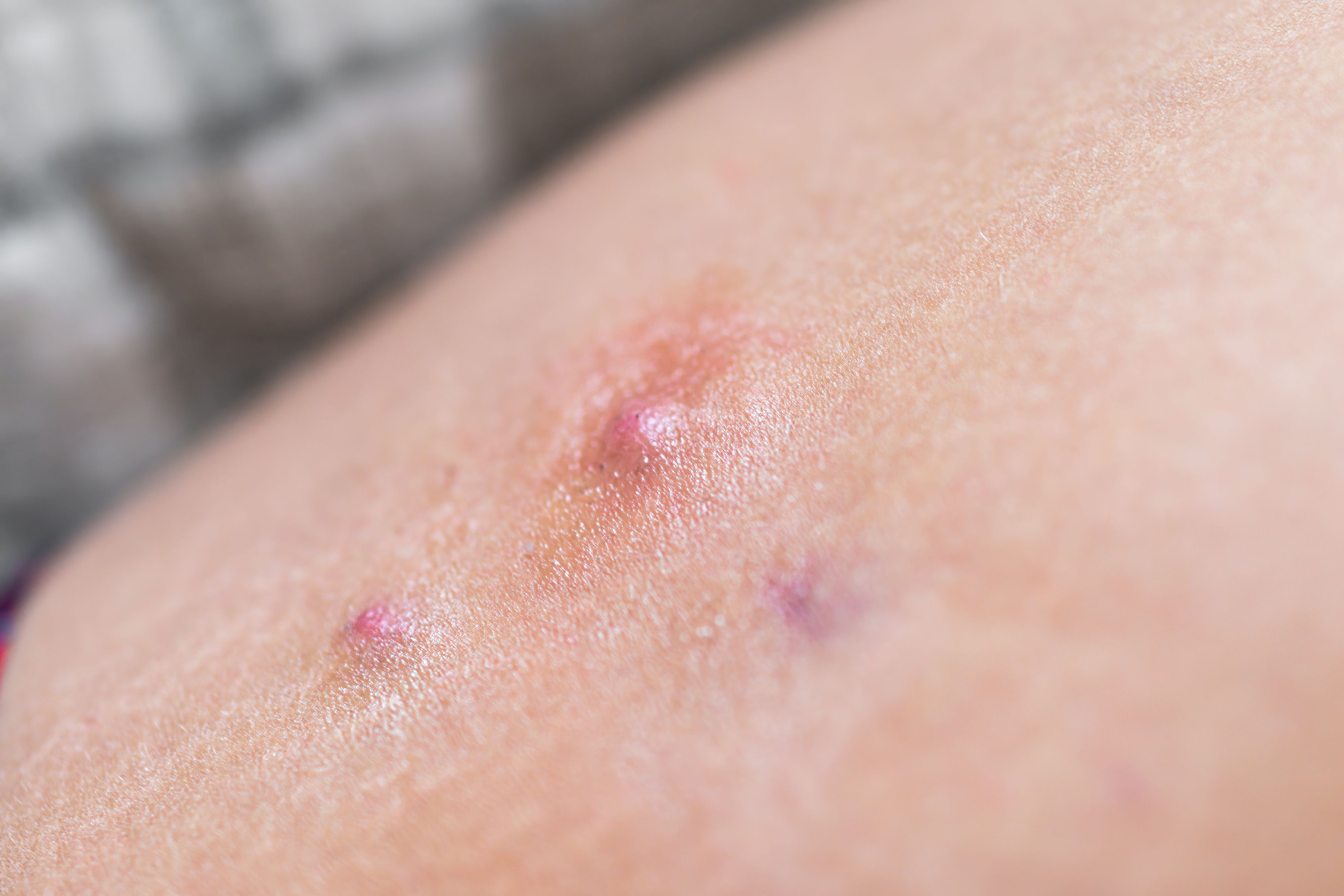
UCB recently presented1 phase 3 data from its BE HEARD I and BE HEARD II phase 3 studies at this year’s European Academy of Dermatology and Venereology Congress, revealing that bimekizumab achieved high thresholds of meaningful clinical responses in patients with hidradenitis suppurativa (HS).
Earlier this year, UCB shared results from the studies.2
Read Dermatology Times’ previous coverage of the BE HEARD studies here.
Key Takeaways
- UCB's bimekizumab showed significant clinical responses in patients with hidradenitis suppurativa (HS) in phase 3 studies, achieving HiSCR 50 in over 55% of patients by week 16, surpassing the placebo group's 33.4% response rate.
- By week 48, more than 60% of patients treated with bimekizumab achieved HiSCR75, and approximately 30% reached HiSCR100, with consistent clinical response superiority over placebo across various patient factors.
At baseline, approximately 83.7% to 88.4% of patients had classified severe HS. By week 48, 37.3% to 40.1% had mild HS, and 23.8% to 25.3% had moderate HS.
The pooled data analysis revealed high rates of treatment response among patients with HS as early as week 16. At week 16, 58% of patients receiving bimekizumab Q2W/Q2W, 55.9% Q2W/Q4W, and 56.1% Q4W/Q4W achieved the primary clinical endpoint of Hidradenitis Suppurativa Clinical Response (HiSCR) 50. These results were significant when compared with participants receiving a placebo, of which 33.4% achieved HiSCR at the same time. These improvements were increased in approximately 8 out of 10 patients achieving HiSCR by week 48.
By week 48, approximately 6 in 10 patients treated with bimekizumab achieved HiSCR75, and approximately 3 in 10 patients receiving bimekizumab achieved HiSCR100. Responses among patients treated with bimekizumab were consistently higher than those receiving a placebo. Higher proportions of clinical response were achieved in patients treated with bimekizumab versus placebo, regardless of factors such as body mass index and weight categories.
Furthermore, more than 8 in 10 patients across all bimekizumab treatment groups had achieved a count of 0, 1, or 2 for abscess and inflammatory nodule counts at week 16. These count responses had been sustained through week 48 of the studies.
The safety profile of bimekizumab was consistent with previous studies. Treatment-emergent adverse events included hidradenitis, oral candidiasis, and coronavirus infection.
"The bimekizumab Phase 3 clinical trial program in hidradenitis suppurativa included the more stringent clinical outcomes of HiSCR75, HiSCR90 and HiSCR100 in addition to the standard HiSCR50,” said Christos C. Zouboulis, MD, president of the European Hidradenitis Suppurativa Foundation, in a press release1 from UCB. Zouboulis is also director of the departments of Dermatology, Venereology, Allergology and Immunology at Städtisches Klinikum Dessau and the founding professor of Dermatology and Venereology at the Brandenburg Medical School in Germany.
“In these studies, bimekizumab demonstrated clinical meaningful improvements for these outcomes over placebo at Week 16, with improvements increasing for patients remaining in the studies through Week 48,” Zouboulis said. “In addition, improvements in disease severity were seen over time, with the majority of patients with severe hidradenitis suppurativa at baseline shifting to mild-to-moderate disease according to the IHS4 dynamic classification system.”
References
- UCB. Phase 3 data analysis presented at EADV 2023 showed bimekizumab achieved high thresholds of clinical response in Hidradenitis Suppurativa. PR Newswire: press release distribution, targeting, monitoring and marketing. October 12, 2023. Accessed October 12, 2023. https://www.prnewswire.com/news-releases/phase-3-data-analysis-presented-at-eadv-2023-showed-bimekizumab-achieved-high-thresholds-of-clinical-response-in-hidradenitis-suppurativa-301955218.html.
- BIMEKIZUMAB phase 3 data in hidradenitis suppurativa show clinically meaningful, deep and maintained response over 48 weeks. UCB. March 18, 2023. Accessed October 12, 2023. https://www.ucb.com/stories-media/Press-Releases/article/Bimekizumab-Phase-3-Data-in-Hidradenitis-Suppurativa-Show-Clinically-Meaningful-Deep-and-Maintained-Response-over-48-Weeks.