- Dermatology Times, May 2021 (Vol. 42, No. 5)
- Volume 42
- Issue 5
Tirbanibulin Safe, Effective for AK on Face, Scalp
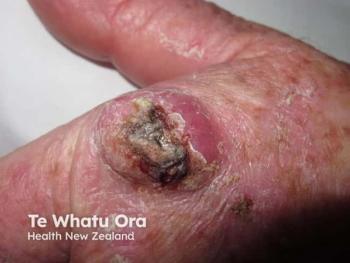
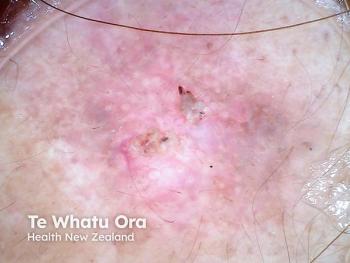
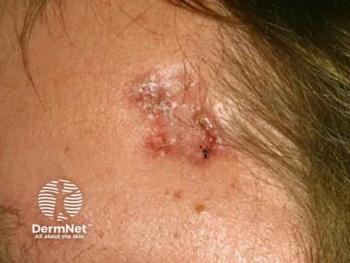
Topical microtubule inhibitor, tirbanibulin (Klisyri, Almirall), demonstrates positive results for treating actinic keratosis (AK) of the face and scalp, according recently published data from two phase 3 studies.
Two recent, identical phase 3 studies showed tirbanibulin 1% ointment applied once daily for 5 days (Klisyri; Almirall) achieved significantly better outcomes compared with vehicle in clearing actinic keratosis (AK) on the face and scalp. Results of the studies evaluating tirbanibulin for the topical treatment of AK in these areas in the New England Journal of Medicine (NEJM)1, according to a company news release.2
This novel microtubule inhibitor approved by the FDA in December 20203 for the topical treatment of AK on the face or scalp was developed in collaboration with Almirall and Athenex, Inc. Beginning in December 2017,4 the strategic partnership between the two companies required Athenex to be responsible for conducting all preclinical and clinical studies (NCT03285477 and NCT03285490) of tirbanibulin in order to gain FDA approval, according to the release. The topical was officially launched in February 2021.5
The recently published studies investigated the safety and efficacy of tirbanibulin 1% ointment (10 mg/g) in 702 adults with AK on the face or scalp in two double-blind, vehicle-controlled, multicenter, parallel-group, randomized clinical trials (KX01-AK-003 and KX01-AK-004). As one of the largest phase 3 clinical study programs ever conducted for a topical AK treatment, study participants were randomly (1:1 ratio) given either tirbanibulin or vehicle, which was self-administered once daily for 5 consecutive days on 25 cm2 of the face or scalp.
Both studies met the primary end point of complete (100%) clearance of AK lesions by day 57 within the face or scalp treatment areas. Notably, each study achieved a highly statistically significant result (P < .0001).
Results of KX01-AK-003 demonstrated a complete (100%) clearance in 44% of patients receiving tirbanibulin compared with 5% of patients treated with vehicle. Meanwhile, in the KX01-AK-004 study, 54% of patients treated with tirbanibulin achieved complete clearance vs 13% vehicle.
Additionally, tirbanibulin achieved the secondary end point in both studies, which was defined as partial (≥ 75%) clearance of lesions (68% of tirbanibulin vs 16% vehicle in KX01-AK-003, and 76% vs. 20% in KX01-AK-004). Results from both studies were highly statistically significant (P < .0001).
“The incidence of actinic keratosis has been increasing, including in younger adults,” said Ayman Grada, MD, head of R&D and medical affairs at Almirall US, in the release. “Patients may prefer a treatment option with a short duration and proven safety and tolerability profile. The data for Klisyri offers this, as well as demonstrating efficacy for both face and scalp actinic keratoses.”
Volker Koscielny, MD, global chief medical officer of Almirall, Barcelona, Spain, added: “The clinical trial data presented not only demonstrates significant efficacy, but importantly a proven tolerability and safety profile. Added to the short, 5-day application period, we believe that Klisyri provides an important addition to the therapeutic armamentarium of US dermatologists in treating actinic keratosis.”
“This important publication represents a significant achievement for Athenex and all of our colleagues who have worked to discover, develop, and bring Klisyri to market,” said Johnson Lau, MD, CEO of Athenex, in the release.
Safety data from both studies was also favorable, with mild-to-moderate local skin reactions occurring and resolving without intervention. They saw no patient withdrawals from the phase 3 studies due to treatment-related adverse events.
Patients with the maximal postbaseline grades for each local skin reaction greater than baseline (> 10%) by treatment group (Klisyri vs vehicle) were: erythema: mild (22%, 28%), moderate (63%, 6%), severe (6%, 0%); flaking/scaling: mild (26%, 25%), moderate (47%, 9%), severe (9%, < 1%); crusting: mild (30%, 9%), moderate (14%, 2%), severe (2%, 0%); swelling: mild (29%, 4%), moderate (9%, < 1%), severe (< 1%, 0%).
“In addition to robust efficacy data, tirbanibulin demonstrated a favorable safety profile,” said Andrew Blauvelt, MD, MBA, president of Oregon Medical Research Center in Spokane, Washington, and one of the lead study investigators. “The most common (≥ 2%) adverse events were local skin reactions, including pruritus and pain, at the application site. No patients withdrew from the study due to treatment-related adverse events.”
Helen Torok, MD, medical director for Dermatology & Surgery Center at Trillium Creek in Medina, Ohio, and a member of the editorial advisory board for Dermatology Times®, said the short treatment time of 5 days and few adverse reactions differentiate this product from other topicals for the treatment of AK on the market.
Those include 5-fluorouracil (Carac; Valeant Pharmaceuticals; Efudex, Mayne Pharma; Fluoroplex, Almirall; Tolak; Pierre Fabre and Hill Dermaceuticals) with a treatment period of 2 to 4 weeks; and imiquimod (Aldara; 3M Pharmaceuticals; Zyclara, Bausch Health Companies) with a treatment period of 4 to 16 weeks, according to Torok.
TREATMENT PROTOCOL
Torok’s typical treatment protocol for AK consists of a 3-step regimen to ensure the field is effectively treated.
“You could do three things [to treat AK on the face and scalp]: topical field treatment, cryosurgery for your hypertrophic actinics, and then photodynamic therapy,” Torok suggested. “That is what really should be done. The whole field should be treated for maximum benefit and maximum results.”
Disclosure:
Torok reported no relevant or financial disclosures.
References:
1. Blauvelt A, Kempers S, Lain E, et al. Phase 3 tirbanibulin for actinic keratosis. N Engl J Med. 2021;384(6):512-520. doi:10.1056/NEJMoa2024040
2. Almirall announces New England Journal of Medicine publication of phase III data demonstrating efficacy and safety of Klisyri (tirbanibulin). News release. PR Newswire. February 11, 2021. Accessed February 11, 2021. https://www.prnewswire.com/news-releases/almirall-announces-new-england-journal-of-medicine-publication-of-phase-iii-data-demonstrating-efficacy-and-safety-of-klisyri-tirbanibulin-301226367.html
3. Athenex announces FDA approval of Klisyri (tirbanibulin) for the treatment of actinic keratosis on the face or scalp. News release. BioSpace. December 15, 2020. Accessed April 12, 2021.
4. Almirall and Athenex announce strategic partnership for the treatment of actinic keratosis. News release. GlobeNewswire. November 12, 2017. Accessed April 12, 2021.
5. Almirall US launches Klisyri (tirbanibulin), a new, innovative topical treatment for actinic keratosis. News release. PR Newswire. February 18, 2021. Accessed April 12, 2021. https://www.prnewswire.com/news-releases/almirall-us-launches-klisyri-tirbanibulin-a-new-innovative-topical-treatment-for-actinic-keratosis-301230528.html
Articles in this issue
over 4 years ago
Administrative Burden of Prescribing Biologicsover 4 years ago
Emerging Biologics Make Waves in ADover 4 years ago
Master Techniques to Treat Eye Issuesover 4 years ago
Current and Upcoming Topicals in the Pipeline for ADover 4 years ago
Off-label Biologics Target Wide Range of Skin Diseasesover 4 years ago
Debunking Sunscreen Controversiesover 4 years ago
Off-label Sarecycline Combats Papulopustular Rosaceaover 4 years ago
What’s Trending in Skin Care? May 2021Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.