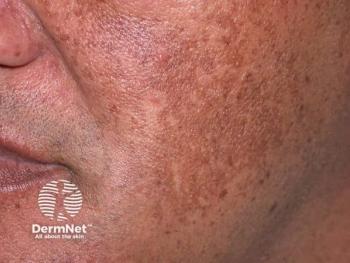
FDA Clears PicoWay Laser for Melasma, Lentigines, and More
The picosecond system has previously been approved for treating acne scarring, pigmented lesions, tattoo removal, and wrinkles.
The
Candela, a Canadian medical device company, announced that the picosecond laser system was approved both by the FDA and by
According to Candela, it is now the
Using photoacoustic energy, Candela said the device is suitable for accessing pigmentation in the epidermis without putting patients at risk of overheating the skin.
Candela also described the device as the laser of choice for patients with darker skin types, which experience an upwards of 50% prevalence rate in comparison to the condition’s standard 1% prevalence.2
“Slower, lower-pulsed lasers, or those that do not consistently deliver advertised energy levels or pulse durations, place this demographic at higher risk of hypo- or hyperpigmentation that can result from excess heat delivery,” said the press release.
Additionally, the laser features four picosecond wavelengths and three beam technologies, including full beam, microbeam, and a fusion beam.
Researchers said this new clearance may pave a new way forward for melasma treatment, which dermatologists have described as
“The PicoWay system's newest clearances allow our customers to continue benefiting from their partnership with Candela and offer their patients another clinically proven option for the treatment of melasma," said CEO of Candela Geoff Crouse in the press release.
Previous management options for melasma include
"Addressing melasma with the PicoWay laser gives patients an important treatment modality to manage this difficult and often psychologically upsetting condition,” said Douglas Wu, MD, PhD in the press release. “Patients now have a treatment that specifically targets their pigmentary changes and can be used alongside other treatments like topicals to better manage this complex skin disorder."
References
- Candela. Picoway® Laser, the picosecond platform with unparalleled versatility and the broadest range of indications, gains new uses, including the challenging condition of Melasma. PR Newswire: press release distribution, targeting, monitoring and marketing.
https://www.prnewswire.com/news-releases/picoway-laser-the-picosecond-platform-with-unparalleled-versatility-and-the-broadest-range-of-indications-gains-new-uses-including-the-challenging-condition-of-melasma-301765289.html . Published March 8, 2023. Accessed March 8, 2023. - Sara Moradi Tuchayi MD. Melasma: What are the best treatments? Harvard Health.
https://www.health.harvard.edu/blog/melasma-what-are-the-best-treatments-202207112776 . Published July 11, 2022. Accessed March 8, 2023. - Ellenberg C. The ultimate guide to treating Melasma. Vogue.
https://www.vogue.com/article/how-to-treat-melasma . Published October 20, 2022. Accessed March 8, 2023.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.