- Dermatology Times, September 2022 (Vol. 43. No. 9)
- Volume 43
- Issue 9
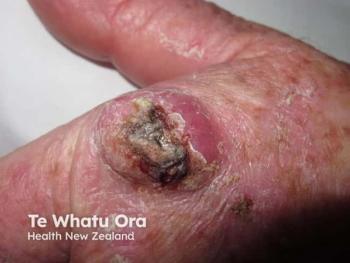
Phase I/II Study of Topical Biologic Shows Positive Results for the Treatment of Basal Cell Carcinoma
MW005 treatment for low-risk basal cell carcinoma would offer patients an alternative to surgical excision.
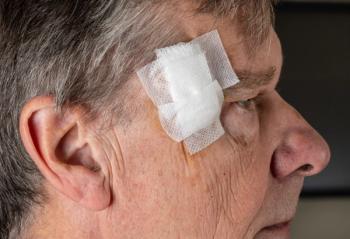
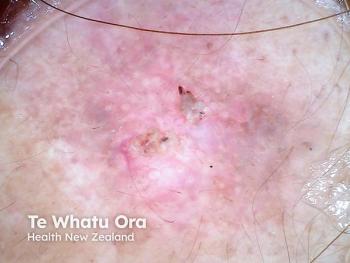
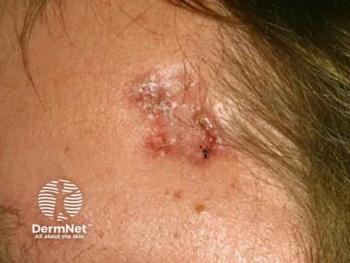
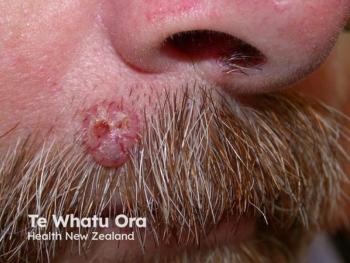
MediWound Ltd. announced positive initial data from a US phase I/II study of MW005, a topical biologic, for the treatment of low-risk basal cell carcinoma (BCC).1 The study included 11 patients in a continuous open-label study. Many of the patients that completed the study achieved complete histological clearance of their target lesions following the use of MW005. Eleven patients in the first cohort with either superficial or nodular BCC were treated. The patients that enrolled in the study were given seven topical applications of MW005 once a day, every other day.
The Phase I/II open-label single-arm study measured the safety and efficacy of MW055 in BCC by monitoring different administration schedules. At the end of the treatment course, patients were monitored for 8 weeks to assess the healing status of their lesions and any adverse effects. After 8 weeks of the post-treatment period, all patients had their targeted lesions excised. Specimens of the BCC lesions were then collected for an independent histological examination. The data collected suggests that MW005 is safe and well-tolerated among patients. It is also designed to make it easier to self-administer.
MW005 is a topical biologic drug and a concentration of proteolytic enzymes enriched in bromelain. The development of MW005 is supported by data found in several toxicological and other preclinical studies. In a 2021
Basal cell carcinoma is a non-melanoma skin cancer that develops from the basal layer of the epidermis and surrounding area. MediWound’s press release stated, “According to the American Cancer Society, BCC is the most diagnosed skin cancer in the United States with approximately 4.3 million cases diagnosed every year. The increasing number of diagnosed BCC is a result of better skin cancer detection, increased sun exposure, and greater life expectancy.” There is a high unmet need for non-surgical treatment options for BCC that still reach high rates of clinical and histological clearance safely.
With the positive results from the phase I/II study of MW005, there is hope that a topical biologic for patients with basal cell carcinoma will be available. Rather than always relying on surgical excisions, patients may be able to find relief through a self-applied topical treatment. MediWound expects to announce the final data of the study in the latter half of 2022.
References:
- Mediwound Ltd. announces positive initial data from its US phase I/II study of MW005 for the treatment of basal cell carcinoma. GlobeNewswire News Room. Published July 11, 2022. Accessed July 20, 2022.
https://www.globenewswire.com/news-release/2022/07/11/2477131/30505/en/MediWound-Announces-Positive-Initial-Data-from-its-U-S-Phase-I-II-Study-of-MW005-for-the-Treatment-of-Basal-Cell-Carcinoma.html - Mediwound Ltd. announces peer-reviewed paper of a case series report of basal cell carcinoma published in the open dermatology journal. GlobeNewswire News Room. Published June 7, 2021. Accessed July 20, 2022.
https://www.globenewswire.com/en/news-release/2021/06/07/2242605/30505/en/MediWound-Announces-Peer-Reviewed-Paper-of-a-Case-Series-Report-of-Basal-Cell-Carcinoma-Published-in-the-Open-Dermatology-Journal.html
Articles in this issue
over 3 years ago
Best Practices for Treating Stretch Marks in Pregnancyover 3 years ago
Creating Diverse Teams Takes Work, But Is Worth the Effortover 3 years ago
The Role of Nonprescription Products in the Management of Acneover 3 years ago
Managing Atopic Dermatitis in Skin of Colorover 3 years ago
Shin Guard Dermatitis: What's the Diagnosis?over 3 years ago
Add an Outpatient Infusion Center to Your Dermatology Practiceover 3 years ago
Can Supplements Effectively Manage Hair Loss?over 3 years ago
Consider Mental Health Concerns in Teens With AcneNewsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.