Off-label uses for fluorouracil cream not always based on strong evidence
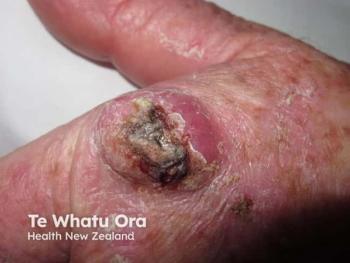
Topical 5‐fluorouracil is FDA-approved to treat actinic keratoses and superficial basal cell carcinoma, but it is commonly used off-label to treat a number of other skin conditions, such as squamous cell carcinoma despite strong evidence.
Topical 5âfluorouracil is FDA-approved to treat actinic keratoses and superficial basal cell carcinoma, but it is commonly used off-label to treat a number of other skin conditions, such as squamous cell carcinoma.
A comprehensive systematic review published in the October issue of the International Journal of Dermatology finds that there is actually little evidence that supports this practice.
“Anecdotally, it appears many clinicians use this treatment as a nonsurgical option for patients and many report a fair amount of success. But there is surprisingly very minimal evidence for the use of topical 5-FU in these lesions,” said Michael Cameron, M.D., a dermatologist with the University of Colorado School of Medicine and corresponding author if the review. “We were hoping to see stronger evidence for the use of 5-fluorouracil in the treatment of squamous cell carcinoma in-situ.”
The literature evidence for squamous cell carcinoma in-situ was limited to case series and two randomized controlled trials comparing topical 5-fluorouracil to photodynamic therapy, which appears to be slightly superior to topical 5-fluorouracil.
“As a tertiary academic center, we encounter many difficult cases that sometimes require us to be creative with our therapies,” Dr. Cameron said in an interview with Dermatology Times. “We were curious to see what the evidence was for off-label uses of topical 5-FU in dermatology.”
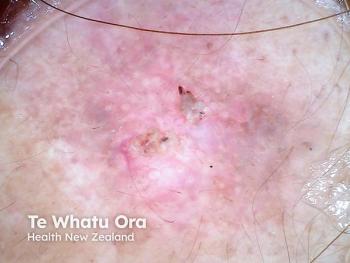
The investigators were not surprised to find that the strongest evidence of topical 5-fluorouracil in dermatology was in its FDA-approved conditions: actinic keratoses and superficial basal cell carcinomas.
THE FINDINGS
In terms of the efficacy of
For example, a double-blinded randomized clinical trial of 19 patients with actinic damage of the arms treated with 0.05 percent tretinoin cream to one arm and a nonreactive vehicle cream to the other arm applied twice daily for two weeks. At three months, the tretinoin-treated arm reduced the incidence of actinic keratoses by nearly a statistically significant amount: 12.3 compared to 10.9 for the control-treated arm. However, 63 percent of patients had more irritation of the arm receiving the dual therapy.
A second study examined the effect of combining low-dose 0.5 percent 5-fluorouracil with 10 percent salicylic acid once daily in a case series of 1,051 patients. At the end of the maximum treatment period of 14 weeks, there was a 69.7 percent reduction in the number of actinic keratoses and an 82.1 percent decrease in size.
A third study found that cryosurgery followed by eight weeks of 0.5 percent 5-fluorouracil once a day was more likely than cryotherapy alone to attain 75 percent clearance: 73 percent versus 43 percent, respectively. Likewise, the combination therapy showed 100 percent clearance 40 percent of the time compared to only 13 percent of the time for cryotherapy alone.
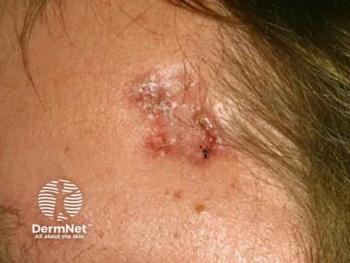
ALTERNATIVE TREATMENTS
Despite the reliability of
For instance, a prospective randomized trial of 75 patients compared clinical, histological and cosmetic outcomes at 12 months for three different treatment regimens: cryosurgery (one or two courses), topical 5 percent 5-FU (twice daily for four weeks) and topical 5 percent imiquimod (three times a week nightly for four weeks).
The results showed that topical imiquimod achieved a better sustained clinical clearance rate: 73 percent compared to 33 percent with 5-fluorouracil and 4 percent with cryosurgery.
The histopathologic clearance rate also favored imiquimod: 73 percent versus 67 with 5-FU and 32 percent with cryosurgery at 12 months.
In addition, imiquimod provided better global cosmetic outcomes.
Another prospective randomized study of 55 patients concluded that a single session of Er:YAG laser resurfacing achieved lower one-year recurrence rates for actinic keratoses than 5 percent 5-FU twice daily for four weeks: 25.9 percent versus 60.0 percent.
“Our review provides a resource for dermatologists to reference when considering off-label uses of topical 5-fluorouracil,” Dr. Cameron says.
A limitation of the review, though, is that it is not a formal meta-analysis.
REFERENCES
Prince GT, Cameron MC, Fathi R, et al. Wenande E, Phothong W, Bay C, et al. “Topical 5-fluorouracil in Dermatologic Disease,” International Journal of Dermatology, 2018, 57: 1259-1264.
DOI: org/10.1111/ijd.14106
Krawtchenko N, Roewert-Huber J, Ulrich M, et al. “A Randomized Study of Topical 5% Imiquimod vs. Topical 5-Fluorouracil vs. Cryosurgery in Immunocompetent Patients with Actinic Keratoses: A Comparison of Clinical and Histological Outcomes Including 1-Year Follow-up,” British Journal of Dermatology, 2007; 157 Suppl 2: 34-40. DOI: 10.1111/j.1365-2133.2007.08271.x
Ostertag JU, Quaedvlieg PJ, van der Geer S, et al. “A Clinical Comparison and Long-Term Follow-Up of Topical 5-Fluorouracil Versus Laser Resurfacing in the Treatment of Widespread Actinic Keratoses,” Lasers in Surgery and Medicine, 2006; 38: 731-739
DOI: 10.1002/lsm.20379
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.