- Dermatology Times, August 2020 (Vol. 41, No. 8)
- Volume 41
- Issue 8
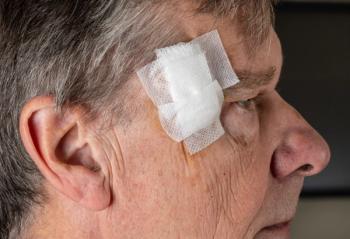
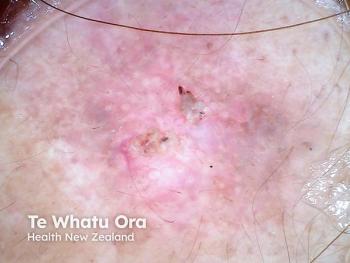
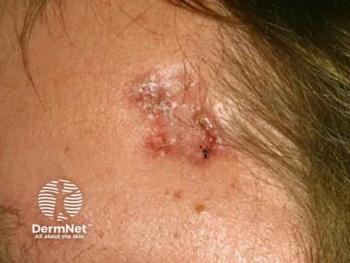
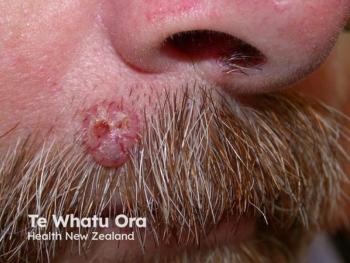
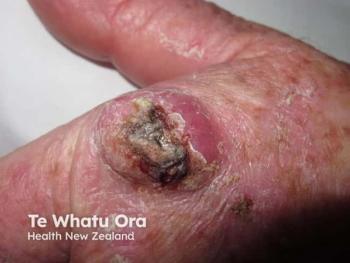
Immune checkpoint inhibitor therapy advances treatment for Merkel cell carcinoma
Treatment for Merkel cell carcinoma has evolved due to the high aggressiveness and metastatic rate of the tumors. Immune checkpoint inhibitor therapy may change prognosis and quality of life.
Merkel cell carcinoma (MCC) is a cutaneous neuroendocrine tumor with a high rate of metastasis and few effective therapeutic options. Continued research has led to the development and recent approval of novel immune checkpoint inhibitors for advanced MCC, such as pembrolizumab (Keytruda, Merck) and avelumab (Bavencio, EMD Serano and Pfizer), which have changed the way clinicians approach management of advanced MCC patients.
Patients with localized MCC have typically been treated with surgery followed by radiation therapy; though, chemotherapy largely remained the best alternative for patients with advanced MCC. Responses to chemotherapy were mostly short-lived however, begging the need for more effective treatment options.
“When traditional chemotherapy is used to treat advanced MCC, it can result in a good response rate in the short term, but patients will usually relapse relatively quickly. The checkpoint inhibitors are a definite game-changer in the treatment of advanced MCC,” says Desiree Ratner, M.D., clinical professor, Ronald O. Perelman Department of Dermatology, NYU Skin and Cancer Clinic, New York, who spoke at the ODAC Dermatology, Aesthetic and Surgical Conference in Orlando.
Avelumab is a human monoclonal antibody that targets the protein programmed death ligand PD-L1 and therefore inhibits binding to its receptor PD-1. Formation of a PD-1/PD-L-1 complex is blocked, resulting in the activation of T-cell mediated immune responses against tumor cells.
In the study that led to approval by the U.S. Food and Drug Administration (FDA), Dr. Ratner says, over half of patients responded well to the drug.
“Advanced MCC patients who have already been previously treated with chemotherapy or other systemic treatments but did not respond can particularly benefit from this novel therapy, with about a third of previously treated metastatic MCC patients still alive at three years after treatment with avelumab Immune checkpoint inhibitors like avelumab offer patients with advanced MCC a more effective treatment alternative than any other systemic agent previously available,” Dr. Ratner says.
The treatment for MCC has been evolving due to the high aggressiveness and metastatic rate of the tumor. According to Dr. Ratner, even patients with localized solitary lesions tend to have sentinel lymph node biopsy (SLNB) followed by adjunctive radiation, not only underscoring the aggressivity of the tumor but the need to meet it head-on with effective therapies. Fortunately, the checkpoint inhibitors appear to meet this challenge and are largely viewed as the new standard of care for patients with advanced MCC.
Although immune response modulating therapy can be associated with negative impacts on the patients’ thyroid and adrenal function as well as other types of immune responses unrelated to the tumor, Dr. Ratner says that the overall benefit for patients is nevertheless likely higher with the medication. Clinicians should look at the risk/benefit ratio of each case individually and have a candid discussion with the patient about potential therapeutic options.
“Avelumab is a welcome tool in our armamentarium for advanced MCC. In my opinion, the drug that has really changed the prognosis and quality of life for a considerable number of those unfortunate patients with advanced MCC,” Dr. Ratner says.
Disclosure:
Dr. Ratner reports no relevant disclosures.
Articles in this issue
over 5 years ago
Survey findings inform messaging on UV exposureover 5 years ago
Studies examine ingredient safety over lifetime useover 5 years ago
Pilot study examines facial microbiome diversityover 5 years ago
Topical minocycline foam safe, well-toleratedover 5 years ago
How to eat for healthy skinover 5 years ago
Skin microbiome altered in some rosacea subtypesover 5 years ago
A new business model for dermatologyover 5 years ago
Is Covid-19 a defense to a battery charge?over 5 years ago
A ‘biomarker’ of psychiatric adverse effects with isotretinoin?Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.