FDA Approves Spesolimab-Sbzo Injection for GPP Flares
Boehringer Ingelheim’s first treatment option for adults will help manage burdensome flares.
The US Food and Drug Administration (FDA) has approved Boehringer Ingelheim’s SPEVIGO (spesolimab-sbzo; Boehringer Ingelheim) injection for the treatment of generalized pustular psoriasis (GPP) flares in adults.1 This injection is the first-ever treatment option for GPP flareups. GPP has a large impact on patients’ quality of life and an increased healthcare burden.
"Generalized pustular psoriasis is a life-threatening condition for which there have not been any approved therapies in the US until now. Off label therapies that have been used are unreliably effective; can be slow for a condition that can be dangerous; and have limited benefit. We finally have an approved drug which results in clearing of pustules within days," said Mark Lebwohl, MD, dean for clinical therapeutics and chariman emeritus of the department of dermatology at the Iachn School of Medicine at Mount Sinai in New York City.
Spesolimab is a monoclonal antibody that inhibits interleukin-36 (IL-36) signaling, a main contributor of GPP. Clinical investigators conducted a subgroup analysis of the primary and key secondary endpoints from the Effisayil 1 (NCT03782792) study.2 The study was a multicenter, randomized, double-blind, placebo-controlled trial to determine the safety, efficacy, and tolerability of spesolimab compared to placebo for the treatment of GPP flares.
The primary endpoint was a Generalized Pustular Psoriasis Physician Global Assessment (GPPGA) pustulation sub score of 0, defined as no visible pustules. The key secondary endpoint was the percentage of patients who achieved a GPPGA total score of 0 or 1—clear or almost clear skin.
According to the study, 54% of patients who were treated with spesolimab achieved the primary endpoint vs 6% of placebo. For the key secondary endpoint, 43% of patients treated with spesolimab achieved a GPPGA total score of 0 or 1 compared to 11% of the placebo group.
In Effisayil 1, the most common adverse events (≥5%) in patients treated with spesolimab were asthenia, fatigue, nausea, vomiting, headache, pruritus, prurigo, infusion site hematoma and bruising, and urinary tract infection.
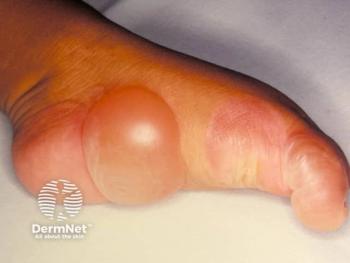
GPP is a rare, life-long, and potentially life-threatening neutrophilic skin disease if left untreated.GPP is clinically distinct from plaque psoriasis and caused by neutrophils (a type of white blood cell) accumulating in the skin, resulting in painful, pus-filled blisters over large areas of the body. If GPP flares are left untreated, they can become life-threatening due to complications such as sepsis and multisystem organ failure.
References:
- FDA approves the first treatment option for generalized pustular psoriasis flares in adults. Boehringer Ingelheim. September 2, 2022. Accessed September 2, 2022.
https://www.boehringer-ingelheim.us/press-release/fda-approves-first-treatment-option-generalized-pustular-psoriasis-flares-adults - Choon, SE, Lebwohl MG, Marrakchi S, et al. Effisayil 1 :multi-center, double-blind, randomised, placebo-controlled, phase II study to evaluate efficacy, safety and tolerability of a single intravenous dose of spesolimab (BI 655130) in patients with generalized pustular psoriasis presenting with an acute flare of moderate to severe intensity. BMJ Open.2021;11:e043666. doi:10.1136/bmjopen-2020-043666
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.