Study Reveals Successful Results for Treatment of Cutaneous T-Cell Lymphoma
Soligenix announces positive phase 3 results from FLASH study for the use of synthetic hypericin to reduce cutaneous T-cell lymphoma lesions.
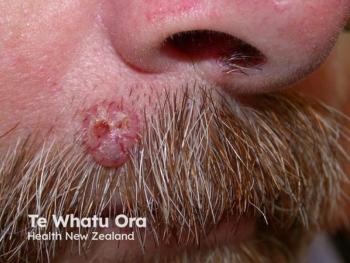
Phase 3 results of the Fluorescent Light Activated Synthetic Hypericin (FLASH) study1 demonstrated significant reductions in cutaneous T-Cell lymphoma (CTCL) lesion sizes with synthetic hypericin (HyBryte; Soligenix) treatment. These findings provide hope for a new treatment to patients diagnosed with the life-altering disease. As a chronic type of cancer that severely impacts patients’ quality of life, there is an unmet need for a well-tolerated and safe treatment option. The FLASH study is the largest double-blind, randomized, placebo-controlled clinical trial for CTCL to date.
The study data also showed that the treatment response continued to improve over 6-week treatment cycles. The main end point of the study evaluated the Composite Assessment of Index Lesion Severity (CAILS) of three specific lesions and the success was determined as ≥50% reduction in CAILS score relative to baseline. Patients reported that their lesions continued to reduce throughout the treatment cycle. After the first 6-week treatment cycle, 16% of patients had a response (p=0.04 versus patients with 6 weeks of placebo treatment). The response rate continued to increase to 49% through 18 weeks of treatment (p<0.0001 versus patients with 6-week hypericin or placebo treatment).
Synthetic hypericin is a novel photodynamic therapy that uses safe, visible light for activation. The drug is topically applied to skin lesions and activated with visible light 16-24 hours after. The US Food and Drug Administration has granted orphan drug and fast track designations for the groundbreaking treatment.2
Data throughout the study indicated that synthetic hypericin was safe and well-tolerated, while current treatment options for CTCL have significant safety concerns and black-box warnings. Synthetic hypericin will offer safer long-term options to patients. CTCL is a class of non-Hodgkin's lymphoma (NHL), a cancer of the white blood cells. Unlike other NIHLs, CTCL is caused by an expansion of malignant T-cell lymphocytes which normally migrate to the skin. There is currently no cure for CTCL.
References
- Kim EJ, Mangold AR, DeSimone JA, et al. Efficacy and Safety of Topical Hypericin Photodynamic Therapy for Early-Stage Cutaneous T-Cell Lymphoma (Mycosis Fungoides): The FLASH Phase 3 Randomized Clinical Trial. JAMA Dermatol. Published online July 20, 2022. doi:10.1001/jamadermatol.2022.2749
- Hybryte cutaneous t-cell lymphoma (CTCL) treatment. Soligenix. Accessed July 29, 2022. https://www.soligenix.com/pipeline-programs/hybryte-for-cutaneous-t-cell-lymphoma-ctcl/
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.