Q1 Accepted NDAs and sBLAs to Keep a Watchful Eye On
Three major NDAs and sBLAs were accepted by the FDA in Q1 of 2023, with PDUFA dates set in 2023 and 2024.
The first quarter of 2023 continued with the ongoing boom in new dermatologic drugs and therapies with the acceptance of 3 new therapies and their Prescription Drug User Fee Act (PDUFA) dates:
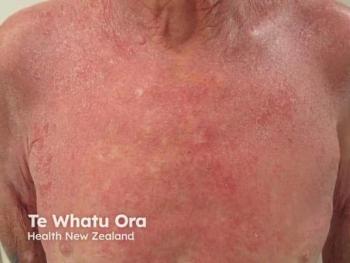
- Verrica Pharmaceuticals’ VP-102 for the treatment of molluscum contagiosum – PDUFA July 23, 2023
- Bristol Myers Squibb’s nivolumab for the treatment of completely resected stage IIB or IIC melanoma – PDUFA October 13, 2023
- Novan Inc.’s berdazimer gel, 10.3% for the treatment of molluscum contagiosum – PDUFA January 5, 2024
VP-102
The US Food and Drug Administration (FDA) accepted Verrica Pharmaceuticals’ resubmitted New Drug Application (NDA) on
Nivolumab
Bristol Myers Squibb’s supplemental Biologics License Application (sBLA) of nivolumab for the treatment of completely resected stage IIB or IIC melanoma was
Berdazimer Gel, 10.3%
Lastly, the FDA approved Novan Inc.’s NDA of berdazimer gel, 10.3% for the
What’s Next?
Regarding the ongoing competition between Verrica Pharmaceuticals and Novan Inc., both hope to provide the first-ever FDA-approved molluscum contagiosum treatment for their patients. Shanna Miranti, MPAS, PA-C, a board-certified physician assistant at Riverchase Dermatology in Naples, Florida, recently spoke to Dermatology Times® regarding the race for VP-102 and berdazimer gel.
“It is a very exciting time in pediatric dermatology right now. We have 2 pharmaceutical companies essentially in a race to the finish to get the very first FDA-approved treatment for molluscum contagiosum,” said Miranti. “I'm sure that a lot of dermatology advanced practice providers like myself will be extremely excited to get our hands on these products. Because right now there's nothing FDA-approved for molluscum. It's amazing to think about how molluscum affects 6 million Americans and over 70% go untreated because they just think that they’re just bumps, or they think they're cosmetic, or because they're not causing any pain, they're not such a problem. But it is a contagious condition. It's something that because I work in pediatric dermatology and general dermatology, I see it every single day.”
As the first PDUFA dates of July 23 for VP-102 and October 13 for nivolumab approach in 2023, it’s crucial for dermatologists and advanced practice providers to keep a watchful eye on FDA approvals and how they can bring these much-needed treatment options to their patients. Follow along with all drug approvals by subscribing to Dermatology Times’
References
- Full indications. Opdivo. Updated August 2022. Accessed March 29, 2023.
https://www.opdivo.com/ - Browning JC, Enloe C, Cartwright M, et al. Efficacy and safety of topical nitric oxide−releasing berdazimer gel in patients with molluscum contagiosum: A phase 3 randomized clinical trial. JAMA Dermatol. 2022;158(8):871–878. doi:10.1001/jamadermatol.2022.2721
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.