Off-label treatments play potential role in management of HOIs
Systemic corticosteroids ormedical intervention is indicated for hemangiomas of infancy. Recenliterature reports describe favorable experience with off-label use of imiquimod cream 5 percent (Aldara, Graceway), becaplermin gel 0.01percent (Regranex, Ortho-McNeil) and vincristine.
Key Points
However, when discussing these modalities with families, it is important to remember that available information on their efficacy and safety is derived mostly from case reports, case series or retrospective studies, and none are Food and Drug Administration (FDA)-approved for the treatment of these vascular tumors, says Amy J. Theos, M.D., assistant professor of dermatology, University of Alabama, Birmingham.
Imiquimod
Subsequent clinical publications included several case reports and case series. More recently, a prospective study including 10 infants and a retrospective study of 22 children have been reported.
Summarizing the published experience, Dr. Theos notes that the typical treatment regimen involved application of a thin layer of imiquimod, three to five times a week, with or without occlusion, for an average duration of 12 weeks. Almost all children developed inflammation and crusting at the application site, which led to temporary discontinuation of treatment. However, no scarring or systemic adverse effects were noted.
"The outcomes achieved suggest imiquimod may speed the natural involution of HOIs. The best results are likely to occur with treatment of uncomplicated, superficial hemangiomas during the proliferative phase, and it is important to be prepared to manage the inflammatory reaction induced by imiquimod and possible ulceration that could ensue," Dr. Theos tells Dermatology Times.
"However, placebo-controlled prospective studies are still desirable to confirm a possible benefit, and pharmacokinetics studies in this young age group are also needed."
Becaplermin gel
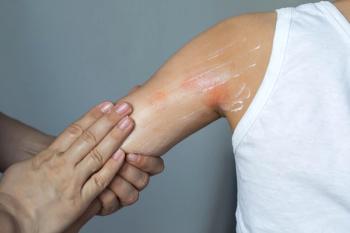
A few papers have described benefits of becaplermin gel for promoting healing of ulcerated HOIs.
The first published report of becaplermin gel described the treatment of a 5-month-old child with an ulcerated plaque-type hemangioma refractory to other topical therapy and oral corticosteroids. Following initiation of a regimen combining becaplermin gel once daily with antibiotics and aggressive wound care, improvement was noted within five days, and the ulcer healed within six weeks.
In 2004, another paper reported, retrospectively, on eight infants with ulcerated perineal hemangiomas that had failed standard treatment with good local wound care and antibiotics. Becaplermin gel was applied once daily followed by a barrier ointment, and all ulcerations healed within three to 21 days.
"There was theoretical concern about using becaplermin to treat HOIs, because it has been shown to stimulate angiogenesis when used to treat chronic ulcers and, thereby, might induce further proliferation of the vascular tumor. In the limited published experience, no proliferation has been observed, even when becaplermin was applied to the entire HOI rather than to the ulcerated area alone," Dr. Theos says.
Vincristine
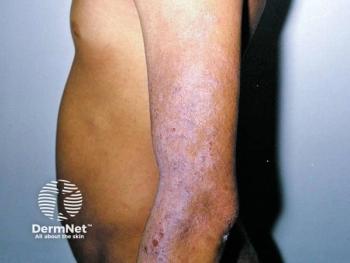
Several case reports exist on the usefulness of vincristine in the management of HOIs that are life-threatening or cause severe functional impairment or disfigurement.
"When second-line treatment for life-or function-threatening HOIs is indicated after systemic corticosteroids fail, vincristine may be preferred over interferon alfa because of its more favorable side effect profile, particularly with a lower risk of spastic diplegia," Dr. Theos says.
Disclosure: Dr. Theos has no financial interest in any of the material she discussed.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.