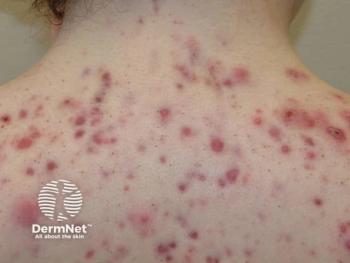
Isotretinoin registry: safety or regulation overkill?
National report — Like isotretinoin itself, the recently mandated patient registry for this drug represents a mixed bag, sources tell Dermatology Times.
National report - Like isotretinoin itself, the recently mandated patient registry for this drug represents a mixed bag, sources tell Dermatology Times.
While none dispute the value of the registry's goal, dermatologists and others worry that the registry ultimately will place onerous restrictions upon a valuable therapy - possibly driving unintended consequences, including black market sales.
In August, the Food and Drug Administration (FDA) announced that by year's end, patients, physicians and providers must enroll in a computerized registry designed to prevent fetal exposure to isotretinoin. The registry is part of a comprehensive program called iPLEDGE, which sets November 1 as the date by which wholesalers and pharmacies must register to obtain isotretinoin from manufacturers. Patients and prescribers have until December 31, 2005, to register and comply with requirements in areas including office visits, counseling and birth control.
Registration particulars
Patients must then register themselves (either online or by phone) as well. When registering, both men and women must acknowledge risks associated with isotretinoin use, including depression and suicidality. Women of childbearing age furthermore must submit results of two CLIA (Clinical Laboratory Improvement Amendments)-certified laboratory pregnancy tests prior to getting an initial prescription written.
The registry process gives women seven days from the date their prescriptions are written to fill them. Additionally, women must submit evidence of a negative pregnancy test monthly before they can get a refill. The system also will schedule female patients for pregnancy tests at the conclusion of therapy and 30 days later.
"Enhancing and combining the previous risk management programs into one collaborative program minimizes possible confusion from having multiple equivalent programs and further tightens existing links between pregnancy testing and dispensing of the drug through mandatory registration of all parties and other mechanisms. The enhancements to the programs strive to prevent, to the extent possible, the risk of fetal exposure to isotretinoin," says a spokesperson for iPLEDGE, whose sponsors include Hoffman-LaRoche (Accutane), GenPharm (Amnesteem), Ranbaxy Pharmaceuticals (Sotret) and Barr Laboratories (Claravis).
Reactions to registry
Dermatologists contacted by DermatologyTimes appear to view the registry as a necessary evil.
"We are concerned about the narrow seven-day window between office visit and pickup of prescriptions, the lack of a 24-7 hotline and availability of personnel to answer the many questions that will arise. However, since the registry is the only way the drug can remain available to patients and prescribers in the United States, the AAD (American Academy of Dermatology) urges that everyone comply with the requirements of the registry," says Barbara R. Reed, M.D., chair of the AAD's isotretinoin task force.
The registry was inevitable, agrees David M. Pariser, M.D., the AAD's secretary-treasurer. However, he says, "We don't believe it's going to solve the stated problem, which is to eliminate pregnancies. It may help some, but we feel it's going to limit access to the drug. The new program has so many more requirements on the part of the provider that it isn't going to be in the private practitioner's interest to take all this time for no additional reimbursement."
On a positive note, the registry could give dermatologists a tool for keeping isotretinoin away from pushy patients seeking a quick fix for mild to moderate acne, says Emil Tanghetti, M.D., a Sacramento, Calif.-based dermatologist and clinical professor of medicine at the University of California, Davis.
"It's going to put some responsibility back on the patient's shoulders," he says. "But for those patients who need (isotretinoin) and physicians who treat them, the registry is another roadblock to delivering care in a cost-effective manner."
Since preventing pregnancy appears to be the registry's main goal, he continues, "Why are we registering male patients? There are apparently some issues with depression or suicidal ideation. But these have never been proven without a doubt. The registry is way overkill."
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.












