Adjuvant Treatment for Merkel Cell Carcinoma
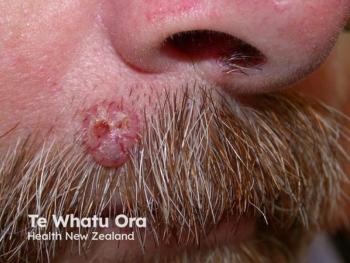
Merkel cell carcinoma is a rare, aggressive cutaneous malignancy that originates due to the uncontrollable proliferation of Merkel cells.
Merkel cell carcinoma (MCC), also called cutaneous neuroendocrine carcinoma or trabecular cancer, is a rare, aggressive cutaneous malignancy that originates due to the uncontrollable proliferation of Merkel cells. The condition tends to grow rapidly and has a high potential for local recurrence and distant metastases.1 Generally, surgery is the standard treatment for Merkel cell carcinoma. However, adjuvant treatment is usually recommended after the primary treatment to control disease recurrence and metastasis.
What is Adjuvant Therapy?
Adjuvant therapy is a treatment given after the primary treatment to reduce the chances of recurrence by killing any remaining tumor cells. The therapy may include chemotherapy, radiotherapy, immunotherapy, hormonal, and targeted therapy.
Adjuvant Therapy Options for MCC Treatment:
Chemotherapy: It exhibits its effects by killing cells that divide quickly. Initially, it can be used as a neoadjuvant treatment to shrink the tumor size. However, the tumor soon develops resistance and regrows despite receiving chemotherapy, making it more challenging to treat due to chemotherapy-induced immune suppression. Adjuvant chemotherapy is not recommended to treat MCC due to possible side effects. For instance, most patients undergo resistance to chemotherapy, making the tumor less responsive to palliative chemotherapy. Patients also complain of poor treatment outcomes, decreased quality of life, and suppressed immune function; moreover, 60% of patients develop neutropenia, whereas blood infections and fever occur in 40% of patients receiving chemotherapy. It is generally given to patients who do not respond to radiotherapy or immunotherapy and reserved for later stages when the tumor is widespread. Etoposide combined with carboplatin is the most common therapy for patients with advanced MCC.2
Radiotherapy: It destroys the genetic material of cancer cells with the help of penetrating beams of energy waves, thereby making them unable to grow. Data suggest that radiotherapy helps control local and nodal MCC recurrence and may help improve the survival of patients with MCC.1 Radiotherapy is mainly recommended in generally healthy patients with a significant risk of local-nodal recurrence. However, it is not recommended in patients with tumor size of <1cm, negative sentinel lymph node biopsy, with no lymphovascular invasion or chronic immunosuppression. Patients with tumors not located in sites with high recurrence rates, like head and neck, should also not receive radiotherapy. Moreover, common side effects associated with radiotherapy include hair loss in the irradiated area, skin irritation, fatigue, and changes in the color and texture of the skin.3
Immunotherapy: Immunotherapy augments the ability of the body’s immune cells to recognize and destroy tumor cells, providing 50% clinical benefits.4 It exerts long-lasting effects by blocking the PD-1 pathway. Among the therapies used, immune checkpoint inhibitors (ICIs) serve as the most promising treatment option. Several ICIs, including Pembrolizumab and Avelumab, have been approved to treat advanced MCC. In 2017, NCCN guidelines listed Pembrolizumab as a treatment option for patients with metastatic MCC. However, in 2018, the drug was approved by the FDA to treat the tumor. Other ICIs, including nivolumab and ipilimumab, are under investigation in clinical trials for advanced MCC. Immunotherapy is generally recommended for patients with advanced disease, distant metastasis (stage IV), or diseases that cannot be treated surgically. In contrast, it should not be given to immunosuppressed patients. Clinical trials for phase III ADAM trial with adjuvant Avelumab, phase III STAMP study and phase II ADMEC-O trial with adjuvant nivolumab are under investigation. Although it produces promising results, common side effects of the treatment approach include nausea, fatigue and itching. ICIs can often cause life-threatening conditions due to immune attacks on the body’s normal cells, such as hepatitis, pneumonitis, never damage, etc.5
References
- Petrelli F, Ghidini A, Torchio M, et al. Adjuvant radiotherapy for Merkel cell carcinoma: A systematic review and meta-analysis. Radiother Oncol. 2019;134:211-219. doi:10.1016/j.radonc.2019.02.015
- Chemotherapy for Merkel cell carcinoma. Merkel Cell Carcinoma. https://merkelcell.org/treatment/chemotherapy/. Published January 16, 2021. Accessed December 16, 2022.
- Radiation therapy for Merkel cell carcinoma. Merkel Cell Carcinoma. https://merkelcell.org/treatment/radiation-therapy/. Published January 16, 2021. Accessed December 16, 2022.
- Tanda ET, d'Amato AL, Rossi G, et al. Merkel Cell Carcinoma: An Immunotherapy Fairy-Tale?. Front Oncol. 2021;11:739006. Published 2021 Sep 23. doi:10.3389/fonc.2021.739006
- Immunotherapy treatment for Merkel cell carcinoma. Merkel Cell Carcinoma. https://merkelcell.org/treatment/immunotherapy/. Published January 16, 2021. Accessed December 16, 2022.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.