- General Dermatology
- Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
Mallinckrodt Receives FDA Label Update for StrataGraft Xenotransplantation Requirements
The FDA no longer restricts the donation of human cells, tissues, cellular or tissue-based products, human milk, ova, sperm, or organs for transplantation for patients who receive StrataGraft.
Image courtesy of Mallinckrodt
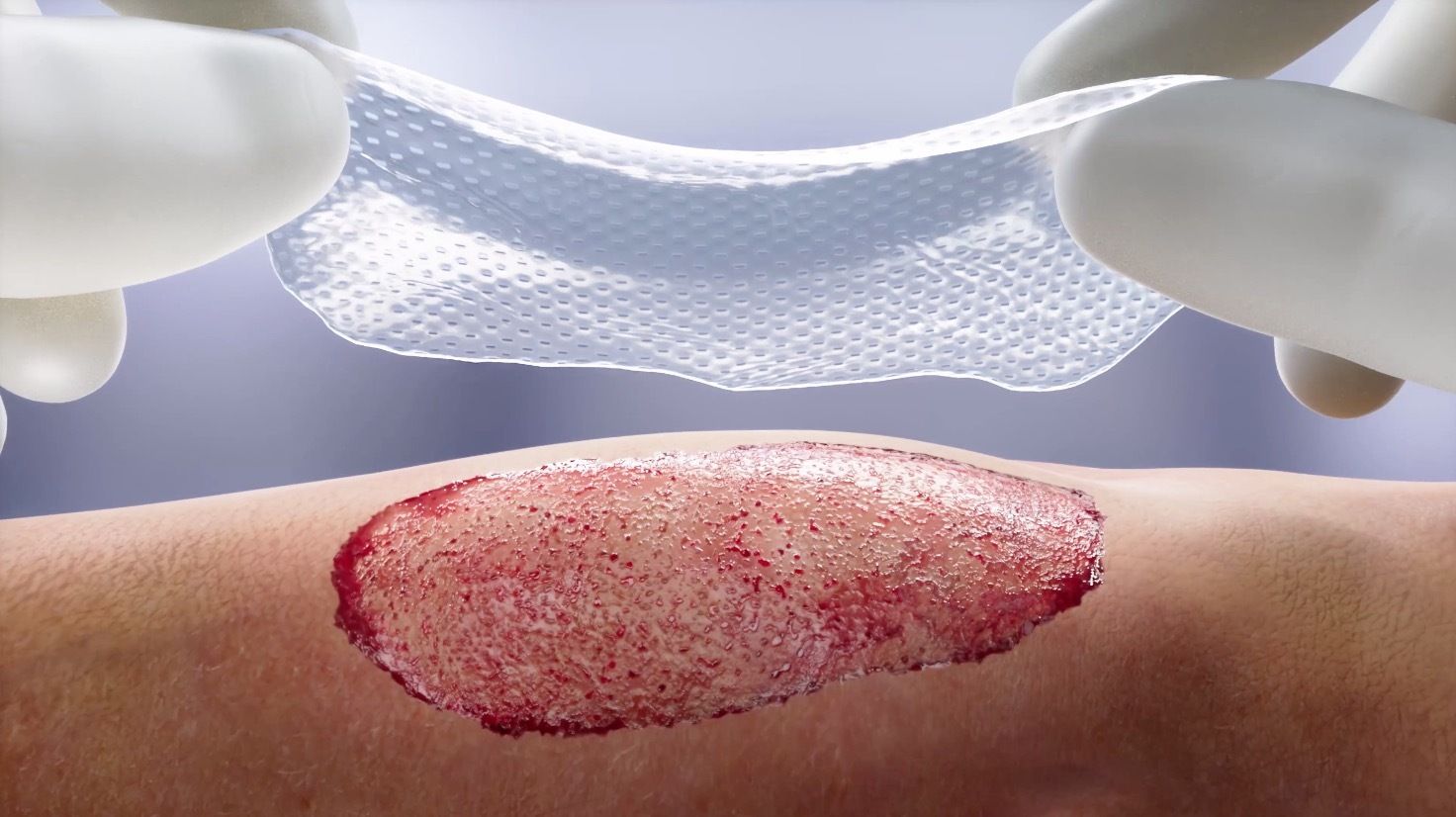
Mallinckrodt Pharmaceuticals recently announced the updated US Food and Drug Administration (FDA) label of its bioengineered StrataGraft (allogeneic cultured keratinocytes and dermal fibroblasts in murine collagen –dsat) to reduce xenotransplantation requirements. The FDA no longer restricts the donation of human cells, tissues, cellular or tissue-based products, human milk, ova, sperm, or organs for transplantation for patients who receive StrataGraft. Additionally, the FDA no longer requires Mallinckrodt or hospitals to collect or store blood samples or patient information.1
“The StrataGraft label update reflects FDA's acknowledgment of reduced xenotransplantation risks and marks a pivotal advancement for the burn care community. Eligible patients who receive StrataGraft can now donate human cells, tissues, cellular or tissue-based products (HCT/Ps), human milk, ova, sperm, or organs for transplantation. Although restrictions still exist around donation of whole blood, blood components, plasma or leukocytes, individual blood collection centers may apply for a waiver of these restrictions for StrataGraft recipients. This restriction is not specifically imposed on StrataGraft recipients but rather on all xenotransplant recipients,” Allen Comer, PhD, the senior director of research at Stratatech Corporation, a Mallinckrodt company, told Dermatology Times. “For commercial use, the FDA no longer requires Mallinckrodt to collect patient information and blood samples and maintain a patient database. This applies to patients treated under the commercial program, but not for clinical trial subjects. This update helps alleviate the patient burden of continual blood testing and personal health information sharing.”
Image courtesy of Mallinckrodt
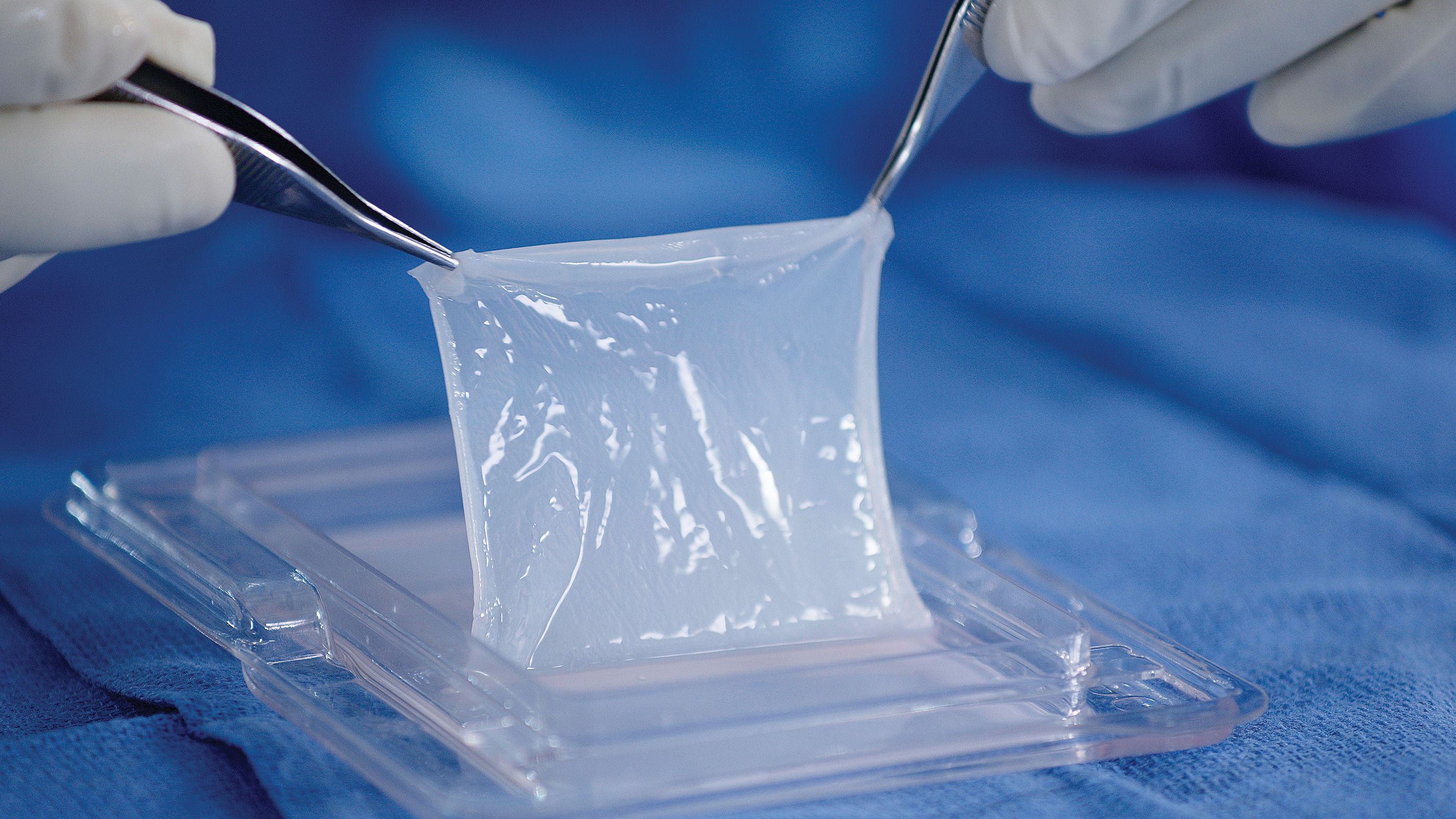
According to Mallinckrodt's news release, more than 40,000 people in the US are hospitalized due to severe burns.2 Autologous skin grafting is the most common inpatient treatment for burn and surgical excision of healthy skin from an uninjured area of the patient’s skin and transplanting the skin graft to the injury.2 The surgical excision of health skin creates a donor site wound and leaves the patient with more wounds and scarring.3 Mallinckrodt’s Evidence Generation and Data Sciences team estimates that of those requiring in-patient care annually, 6000 to 10,000 patients are treated with an autograft.4
Comer provided the following benefits regarding StrataGraft’s unique healing process:
- StrataGraft is the first approved alternative to autograft for the treatment of adults with deep partial-thickness (DPT) thermal burns that does not require a donor site at application.
- StrataGraft delivers viable cells to support the body’s own ability to heal.
- StrataGraft contains metabolically active cells that produce and secrete a variety of growth factors and cytokines. Growth factors and cytokines are known to be involved in wound repair and regeneration.5
- StrataGraft is a single-procedure thermal burn treatment. In the pivotal phase 3 trial, donor site harvest was eliminated for 96% (68/71) of the DPT burn sites treated with StrataGraft. As a result, pain and scarring were significantly reduced at potential donor sites that were spared and remained intact (p<0.0001). 5,6
- Overall, the safety profile of StrataGraftwith regard to wound-related events, including erythema, swelling, local warmth and wound site infections, was similar to that of autografting in clinical studies.5
- The most common (2%) adverse reactions were itching (pruritus), blisters, hypertrophic scars, and impaired healing.5
The FDA granted StrataGraft Orphan Drug Designation, and it was among the first products designated by the FDA as a Regenerative Medicine Advanced Therapy under the provisions of the 21st Century Cures Act. At the time of approval, the FDA awarded Stratatech Corporation, a Mallinckrodt company, a Priority Review Voucher (PRV). The Biomedical Advanced Research and Development Authority (BARDA), which is part of the Office of the Assistant Secretary for Preparedness and Response under contract HHSO100201500027C has funded in part the development of StrataGraft.
StrataGraft was approved by the FDA in 2021 based on data from the pivotal phase 3 STRATA2016 (NCT03005106) clinical trial.7
“Burn surgeons will continue to be able to provide StrataGraft as a potentially effective and less-invasive treatment option for appropriate patients with DPT burns. Burn surgeons can now use StrataGraft without these requirements which impact efficiency in treatment administration and patient monitoring– ultimately contributing to overall improvement in DPT burn management to help improve patient outcomes,” concluded Comer.
For full prescribing and safety information on StrataGraft, click here.
References
- Mallinckrodt receives US Food and Drug Administration label update for StrataGraft (allogeneic cultured keratinocytes and dermal fibroblasts in murine collagen – dsat) reducing xenotransplantation requirements. News release Mallinckrodt Pharmaceuticals. September 5, 2023. Accessed September 11, 2023. https://www.mallinckrodt.com/about/news-and-media/news-detail/?id=30216#_edn2
- Burn incidence fact sheet. American Burn Association. Accessed September 11, 2023. http://ameriburn.org/who-we-are/media/burn-incidence-fact-sheet/
- McDermott KW, Weiss AJ, Elixhauser A. Burn-related hospital inpatient stays and emergency department visits, 2013. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD): Agency for Healthcare Research and Quality (US); December 2016.
- Yu TC, Zhang X, Smiell J, et al. Healthcare resource utilization, treatment patterns, and cost of care among patients with thermal burns and inpatient autografting in two large privately insured populations in the United States. Burns. 2020;46(4):825-835. doi:10.1016/j.burns.2019.10.019
- StrataGraft Package Insert. Stratatech Corporation. https://stratagraft.com/assets/pdfs/StrataGraft-USPI.pdf
- Gibson ALF, Holmes JH IV, Shupp JW, et al. A phase 3, open-label, controlled, randomized, multicenter trial evaluating the efficacy and safety of StrataGraftconstruct in patients with deep partial-thickness thermal burns. Burns. 2021;47(5):1024-1037. doi:10.1016/j.burns.2021.04.021.
- Mallinckrodt announces US FDA approval of StrataGraft (allogeneic cultured keratinocytes and dermal fibroblasts in murine collagen - dsat). News release. Mallinckrodt Pharmaceuticals. June 15, 2021. Accessed September 11, 2023. https://www.multivu.com/players/English/8801751-mallinckrodt-stratagraft/
