- General Dermatology
- Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Anti-Aging
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
Technology targets non-genital warts
Positive results from a study investigating non-thermal Nano-Pulse Stimulation as a treatment for cutaneous non-genital warts have led to a larger multicenter study that is looking to optimize treatment parameters and hopefully replicate the early evidence of its efficacy and safety.
Dr. Munavalli

Positive results from a feasibility study investigating non-thermal Nano-Pulse Stimulation (NPS, Pulse Biosciences) for treating cutaneous non-genital warts have led to a larger multicenter study that aims to optimize the treatment parameters and hopefully replicate the early evidence of its efficacy and safety, says Gilly S. Munavalli, M.D., M.H.S.
RELATED: Can treating warts do more harm than good?
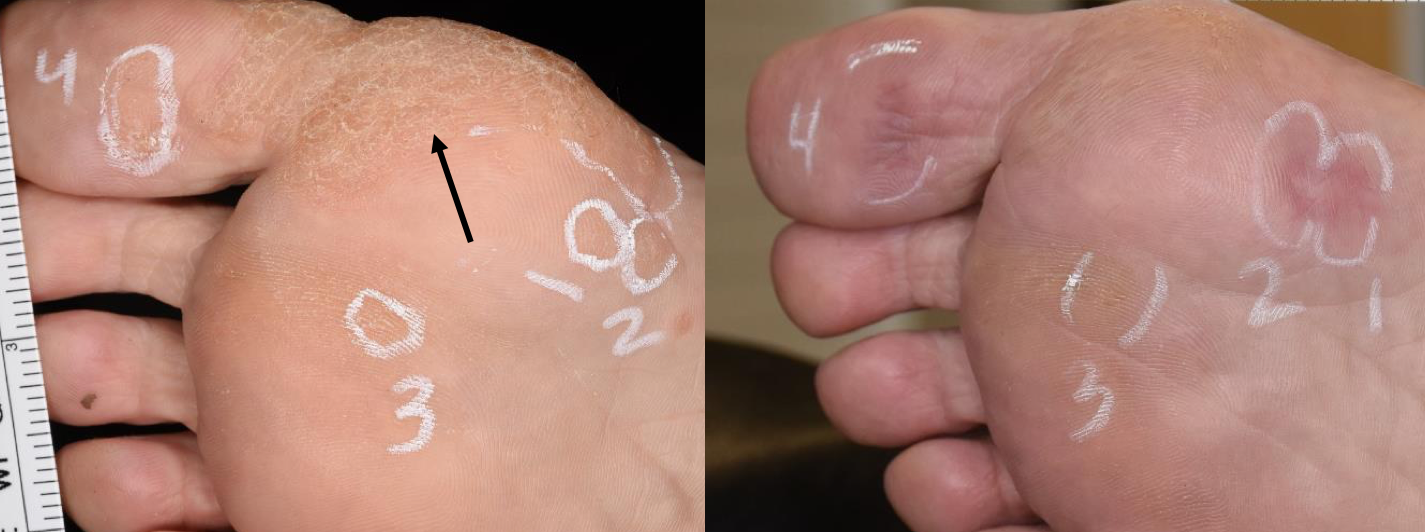
The initial study enrolled 19 patients, of which a sizeable proportion had warts considered “difficult-to-treat” based on their location or failure to be cleared by other modalities. Even so, clinical and dermoscopic assessments showed that approximately 60% of treated lesions were cleared after one or two treatments with NPS.
Performed under local anesthesia, the NPS treatment was well-tolerated and associated with generally favorable cosmetic results. Impressively, clearance was also noted for many untreated, non-contiguous warts, reported Dr. Munavalli, assistant clinical professor of dermatology, Wake Forest University School of Medicine, Winston-Salem, N.C., and founder and medical director, Dermatology, Laser and Vein Specialists of the Carolinas, Charlotte, N.C.
“Patients seek treatment for non-genital warts because they can cause discomfort and, if visible, carry a social stigma. Some warts, particularly plantar warts, can be very refractory to our currently used treatment approaches,” he says.
“The results from the first human investigation of NPS treatment for non-genital warts are encouraging. Now we look forward to analyzing data from the larger study and the possibility of having another option to treat what can be a bothersome problem.”
Dr. Munavalli and Vic Ross, M.D., Scripps Clinic, San Diego, Calif., were co-investigators in the initial feasibility study. Eligible patients could have up to two warts treated and needed to have a third wart that could be left untreated as a control. They were seen for follow-up after seven, 30 and 60 days.
RELATED: Wart treatment clinical trials hold promise
Reduction in wart size was often seen at the 30-day visit, and tissue recovery at sites of cleared warts was rapid and complete. The treatment was associated with immediate pinpoint bleeding and swelling and sometimes hemorrhagic blisters and crust formation.
“These local reactions were all self-limited and similar to what we see with other destructive technologies,” says Dr. Munavalli.
“However, because the treatment zone with other options, such as cryotherapy, is not very controllable, they can cause damage to surrounding skin. In contrast, the effect of NPS is very specific to the area covered by the treatment tip, and in this study, we had positive patient reports of a minimal pain recovery period.”
The NPS treatment was associated with some residual erythema that tends to fade over time, as well as a low incidence of hyperpigmentation, which may be reduced through future re nement of the treatment parameters.
“Overall, patient satisfaction has been very high, which is not surprising considering that our cohort included patients who were probably frustrated after failing other therapies,” says Dr. Munavalli.
BIOLOGICAL BASIS FOR EFFICACY
NPS involves the delivery of low energy, high voltage, ultra-short (nanosecond) electrical pulses to the targeted lesion that ultimately results in regulated apoptotic cell death. It is postulated that the destruction of the lesion cells is accompanied by extracellular release of human papilloma viral specific antigens that subsequently trigger an immune response.
RELATED: Nano-pulse stimulation promising for skin lesions
The observed resolution of untreated warts – a phenomenon that Dr. Munavalli refers to as a bystander effect – supports the idea that the mechanism of action of NPS for clearing warts involves activation of the host immune system.
“This immunomodulatory mechanism of action has been reported in the literature and through clinical observation with other destructive technologies and may not be unique to NPS. With NPS, however, there is a clear basis for understanding how immune activation can occur,” says Dr. Munavalli.
He adds that another important feature of NPS is that it is specific to cellular targets.
“NPS does not affect the dermal connective tissue, hair follicles, or blood vessels, and that is why we generally see good cosmetic results.”
EXPANDING THE EVIDENCE
Three more dermatologists are joining Dr. Ross and Dr. Munavalli as investigators in the second study investigating NPS for the treatment of non-genital warts. They aim to enroll 60 subjects across their ve sites. Patients will be able to receive a total of four NPS treatments with a 30-day interval between treatments.
RELATED: Verrica develops a solution for common warts
As of October 2019, 33 patients were enrolled, and the majority of their warts had been refractory to prior treatments.
The treatments are being performed with one of five tip sizes, depending on wart size. A range of treatment settings are being evaluated with the aim of finding parameters that provide the best benefit:risk ratio.
Disclosures:
Drs. Munavalli and Ross are investigators and members of the scientific advisory board for Pulse Biosciences.

