- General Dermatology
- Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Anti-Aging
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
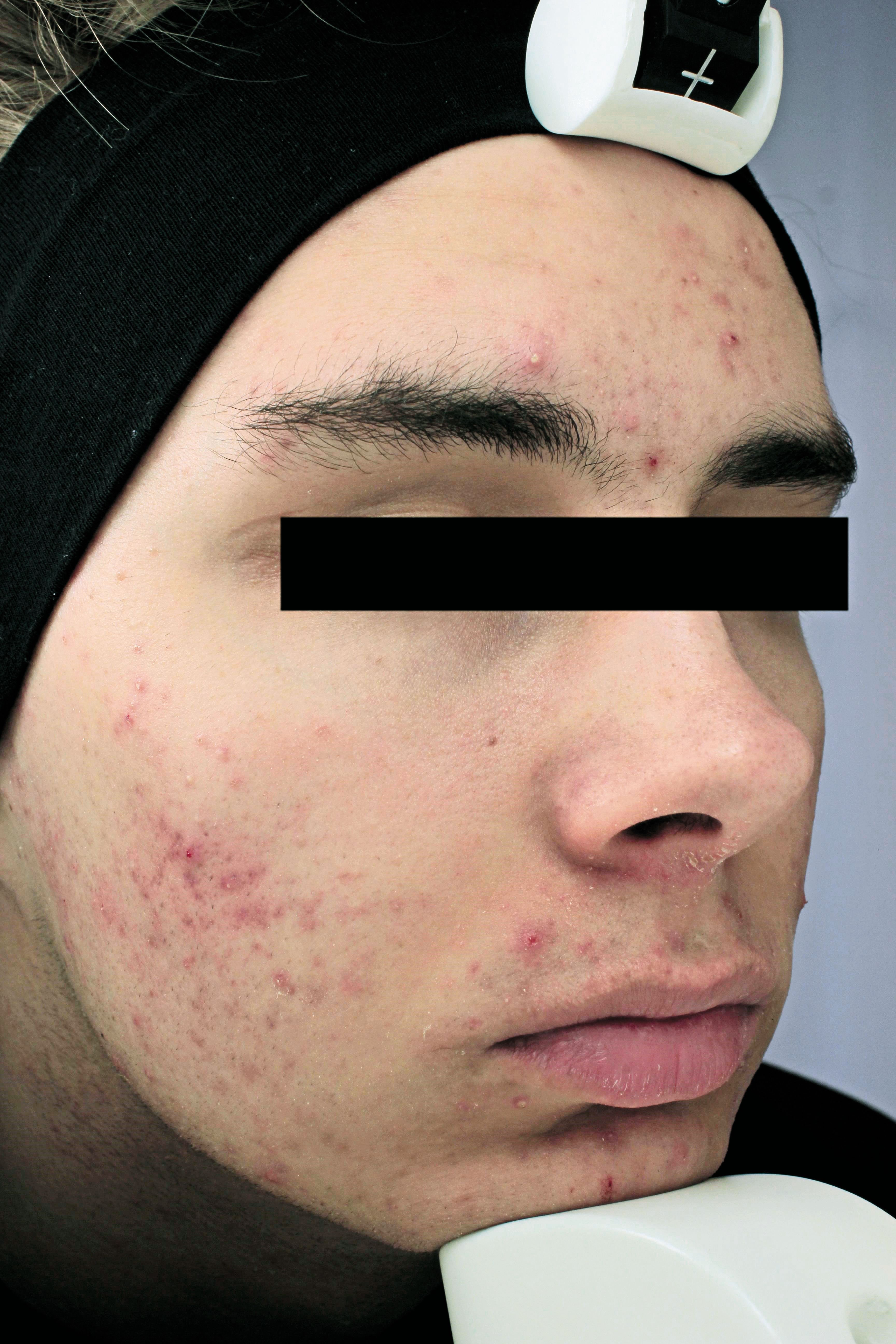
FDA approves Sebacia for acne treatment
The U.S. Food and Drug Administration has approved clearance for a new acne treatment that selectively targets sebaceous glands, according to a news release.

The image shows a patient at baseline. (Photo courtesy of Sebacia Inc.)
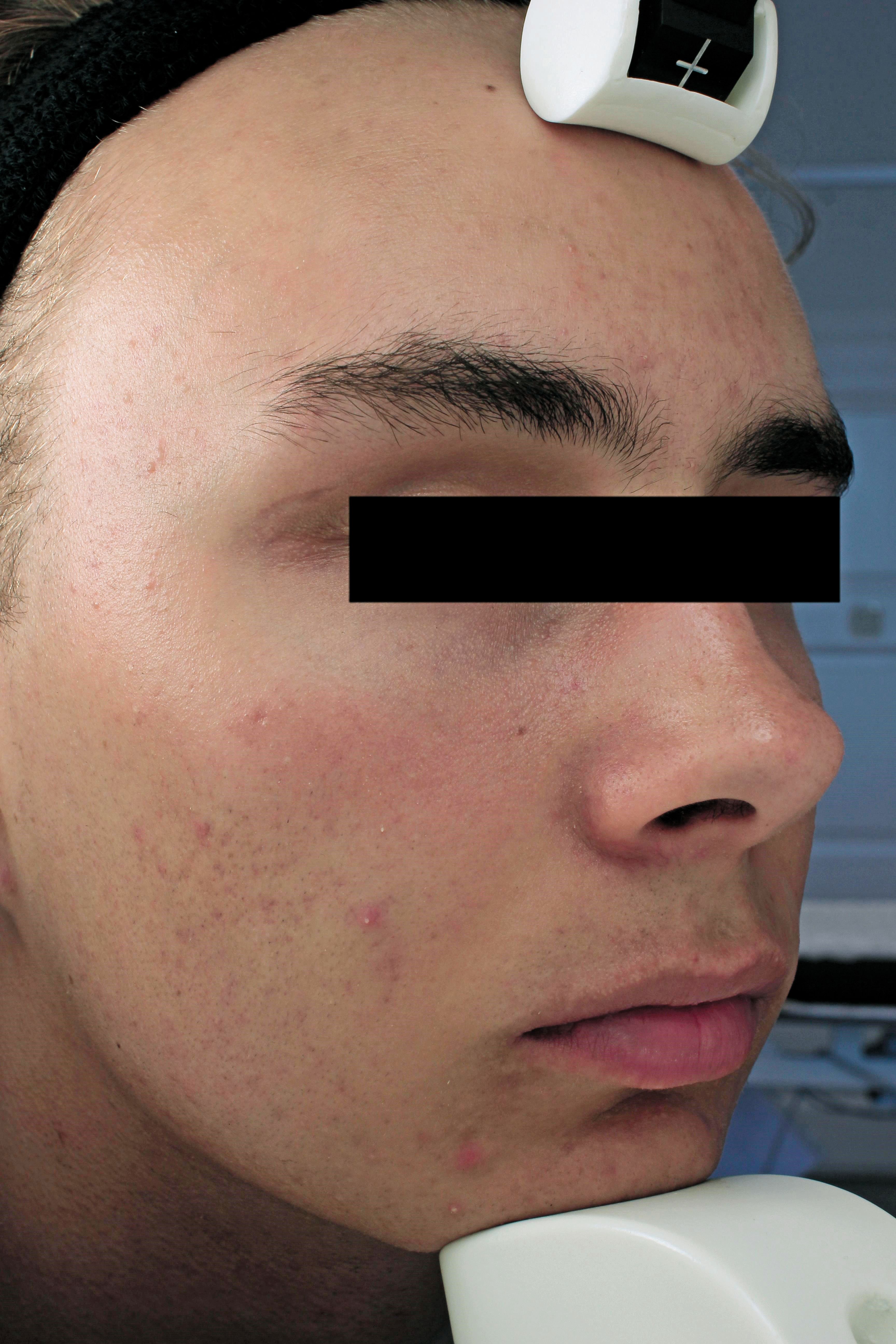
The image shows a patient at 12 weeks post treatment with Sebacia Microparticles monotherapy. (Photo courtesy of Sebacia Inc.)
The U.S. Food and Drug Administration has approved clearance for a new acne treatment that selectively targets sebaceous glands, according to a news release.
Sebacia Microparticles is indicated for use with 1,064 nm lasers, which use photothermal heating of the sebaceous glands to treat mild-to-moderate acne vulgaris.
The product, made by Sebacia Inc., was approved following a randomized, controlled, blinded trial of 168 patients with mild-to-moderate acne. The patients received treatment with laser only or with laser and Sebacia. Those treated with Sebecia Microparticles achieved a 53 percent median reduction in inflammatory lesion count compared to 45 percent of those treated with laser alone, according to the company. At 12 weeks, the study reached its primary endpoint of demonstrating noninferiority. Additionally, 30.1 percent of patients treated with Sebacia Microparticles demonstrated a clear or almost-clear Investigator’s Global Asssessment score.
“We have not seen any truly innovative acne therapies developed for more than two decades and with this clearance, Sebacia Microparticles offers a new option for the millions of mild-to-moderate acne sufferers,” Jill S. Waibel, M.D., board-certified dermatologist, Miami Dermatology and Laser Institute, said in a statement. She served as a Sebacia clinical trial investigator. “I expect Sebacia Microparticles to further enhance patient and physician optionality while seamlessly integrating into the AAD-recommended polytherapeutic approach to managing acne.”
With the FDA approval and its CE marking, Sebacia Microparticles is now cleared for sale in both the U.S. and the EU.

