- General Dermatology
- Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
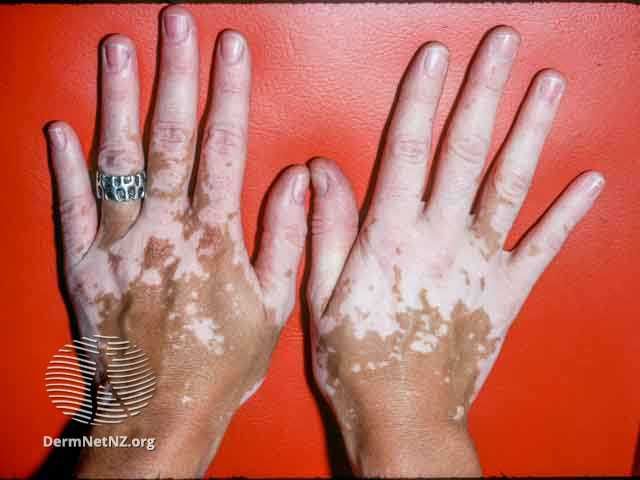
VYNE Therapeutics Provides Updates on VYN201 for Nonsegmental Vitiligo and VYN202 for Inflammatory Diseases
VYNE expects to enroll the first patient with vitiligo in the phase 2b trial for VYN201 in the second quarter of 2024.
VYNE Therapeutics recently announced new pipeline updates on VYN201 and VYN202 for the treatment of nonsegmental vitiligo and other inflammatory skin diseases. The results were announced in VYNE’s first quarter 2024 financial results.1
“During the first quarter, we made steady progress in advancing our VYN201 program toward a phase 2b trial,” said David Domzalski, the president and chief executive officer of VYNE, in the news release. “We are rapidly activating clinical trial sites and expect to dose the first subject in the trial this quarter. In addition, we recently received IND clearance for our VYN202 program to proceed and expect to dose the first healthy volunteers in our phase 1a trial this quarter. We look forward to updating our stakeholders on our progress in the coming months.”
VYN201
Regarding VYN201, a locally administered pan-BD BET inhibitor, VYNE expects to enroll the first patient with vitiligo in the phase 2b trial for VYN201 in the second quarter of 2024. The trial will enroll patients with active or stable nonsegmental vitiligo and will be a randomized, double-blind, vehicle-controlled trial evaluating the efficacy, safety, and pharmacokinetics of once-daily VYN201 gel in 3 dose cohorts of 1%, 2%, and 3% concentrations, compared to vehicle for24 weeks.
Patients in the phase 2b trial will be randomized at a 1:1:1:1 ratio. After the 24-week treatment period, patients in the vehicle group will be equally re-randomized to receive VYN201 1%, 2%, or 3% gel for an additional 28 weeks. VYNE expects to enroll approximately 40 patients in each treatment arm and hopes to report top-line results from the 24-week double-blind part of the trial in mid-2025.
Earlier this year, VYNE announced positive biomarker data from its successful phase 1b trial of VYN201 for nonsegmental vitiligo. The 16-week open-label study included 29 patients with active nonsegmental vitiligo across 3 dose cohorts of 0.5%, 1.0%, and 2.0%. Skin biopsies from selected vitiligo lesions were taken before and after 8 weeks of treatment, revealing promising outcomes. In the 2.0% dose cohort, VYN201 demonstrated significant biological activity, particularly in modulating key biomarkers associated with vitiligo severity and progression.2
VYN202
VYNE’s Investigational New Drug application for VYN202, an oral BD2-selective BET inhibitor, was recently cleared by the US Food & Drug Administration. VYNE announced that it plans to initiate a first-in-human phase 1a single ascending dose/multiple ascending dose (SAD/MAD) trial in healthy volunteers in the second quarter of 2024 and expects to report top line results from the SAD/MAD trial in the second half of 2024.
According to VYNE, if the phase 1a trial is successfully completed, VYNE will initiate phase 1b trials in patients with moderate to severe plaque psoriasis and moderate to severe adult-onset rheumatoid arthritis, with top-line results expected in the second half of 2025.1,3
The VYN202 phase 1a trial is a double-blind, placebo-controlled study that is expected to enroll approximately 64 healthy adult subjects into 5 SAD and 3 MAD cohorts to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of VYN202.3
“VYN202 is an innovative, oral small molecule BD2-selective BET inhibitor that has been designed to achieve class-leading selectivity (BD2 vs. BD1), maximum potency versus BD2, and optimal oral bioavailability. By maximizing BD2 selectivity, VYNE believes VYN202 has the potential to be a more conveniently administered non-biologic treatment option for both acute control and chronic management of immuno-inflammatory indications, in which the damaging effects of unrestricted inflammatory signaling activity is common,” wrote VYNE in a recent news release.3
References
- VYNE reports first quarter 2024 financial results and provides business update. News release. GlobeNewswire. May 9, 2024. Accessed May 9, 2024. https://www.globenewswire.com/news-release/2024/05/09/2878712/0/en/VYNE-Reports-First-Quarter-2024-Financial-Results-and-Provides-Business-Update.html
- Andrus E. Positive biomarker data shared following phase 1b trial of VYN201 in vitiligo. Dermatology Times. January 10, 2024. Accessed May 9, 2024. https://www.dermatologytimes.com/view/positive-biomarker-data-shared-following-phase-1b-trial-of-vyn201-in-vitiligo
- VYNE Therapeutics announces FDA clearance of IND application for VYN202, a novel BD2-selective BET inhibitor. News release. VYNE Therapeutics. May 6, 2024. Accessed May 9, 2024. https://vynetherapeutics.com/press-releases/vyne-therapeutics-announces-fda-clearance-of-ind-application-for-vyn202-a-novel-bd2-selective-bet-inhibitor/