- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
Article
Tildrakizumab Improved Sleep, Quality of Life in Patients With Plaque Psoriasis
Author(s):
The IL-23p19 inhibitor demonstrated long-term efficacy and safety.
In patients with moderate to severe plaque psoriasis, tildrakizumab, an IL-23p19 inhibitor, demonstrated long-term efficacy and safety while improving sleep and quality of life.
Ban/AdobeStock
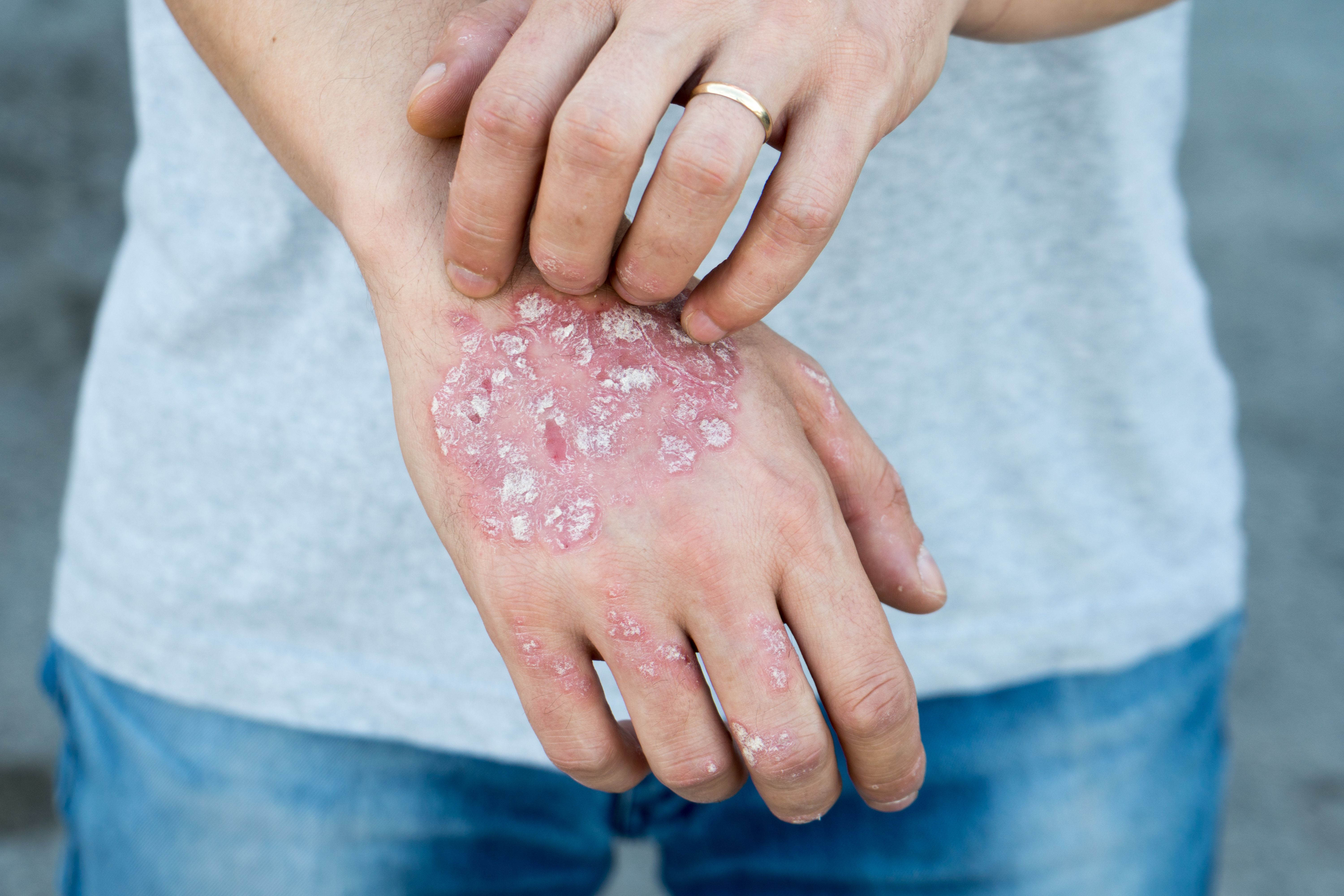
In a study,1 researchers sought to assess the drug’s efficacy in patients with more severe disease in conditions similar to clinical practice, citing its demonstrated safety and efficacy profile in phase 3 trials. According to researchers, recent studies have shown that 78.4% of patients with psoriasis who receive systemic treatments in clinical practice are not and would not be eligible for regulatory randomized controlled trials (RCTs). The study also aimed to characterize the holistic value and benefits of modern psoriasis therapies in clinical settings.
The study, known as TRIBUTE, was an open label phase IV clinical trial that took place in multiple countries and study sites. Participants (n=177) were required to be ages 18 and older and have a moderate to severe chronic plaque psoriasis diagnosis for at least 6 months. For patients who were non-naïve to IL-23 or Th17 pathway inhibitors, researchers required a corresponding washout period.
In total, 113 of 177 patients, or 63.8%, presented with a past medical condition or comorbidities such as hypertension, depression, and asthma at screening.
Participants were subcutaneously injected with 100 mg of tildrakizumab at week 0, 4, and then for every 12 weeksuntil the conclusion of the 24-week study.
Throughout the study, researchers used the Psoriasis Area and Severity Index (PASI) and Dermatology Life Quality Index (DLQI) as primary endpoints to evaluate the drug’s safety and efficacy. Secondary endpoint assessments included:
- Body surface area (BSA)
- DLQI and DLQI-Relevant (DLQI-R)
- Medical Outcomes Study (MOS)-Sleep
- Pain NRS
- PASI
- Patient Benefit Index (PBI)
- Physician’s Global Assessment (PGA)
- Pruritus-Numerical Rating Scale (P-NRS)
- Scaling-NRS
- Skindex-16
- Treatment Satisfaction Questionnaire for Medication (TSQM)
- Work Productivity and Activity Impairment (WPAI)
Using physical examinations, safety laboratories, treatment-emergent adverse events (TEAEs), and vital signs, researchers evaluated the drug’s safety and tolerability.
From baseline to week 24, the average PASI score decreased from 16.2 to 1.0. At week 24, 96% of participants achieved PASI≤5, 88.4% achieved PASI≤3, and 66.5% achieved PASI≤1, with 92.5% achieving PASI 75 and 74.0% achieving PASI 90.
Patients’ BSA decreased from 21.7 at baseline to 1.8 at week 24, and the PGA score decreased from 2.9 at baseline to 0.6 at week 24.
Tildrakizumab also improved participants’ quality of life, with 70.4% of participants achieving DLQI0 and 59.1% achieving DLQI1 by week 24. Skindex-16 scores, on average, decreased from baseline, while proportions of participants achieving Pruritus-NRS, Pain-NRS, and Scaling-NRS scores increased over time.
The MOS-Sleep questionnaire revealed that participants experienced an overall decrease in sleep problems, particularly in the disturbance, adequacy, and somnolence domain. WPAI improved after 24 weeks, with the largest improvement in daily activities.
TEAEs were reported in 29.4% of participants, with the most common events being headache, coronavirus infection, and back pain.
“In conditions close to real clinical practice, TIL (tildrakizumab) improved physical signs of psoriasis, including burdensome symptoms such as pruritus and skin pain after 24 weeks of treatment," study authors wrote. "Moreover, clinically relevant benefits were reported to key aspects of patients’ daily life such as sleep, work, and HRQoL impairment. These findings were accompanied by significant patient treatment benefits and satisfaction as well as a favourable safety profile of TIL."
Reference
- Costanzo A, Llamas‐Velasco M, Fabbrocini G, et al. Tildrakizumab improves high burden skin symptoms, impaired sleep and quality of life of moderate‐to‐severe plaque psoriasis patients in conditions close to clinical practice. J Eur Acad Dermatol. Published online 2023. doi:10.1111/jdv.19229





