- General Dermatology
- Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
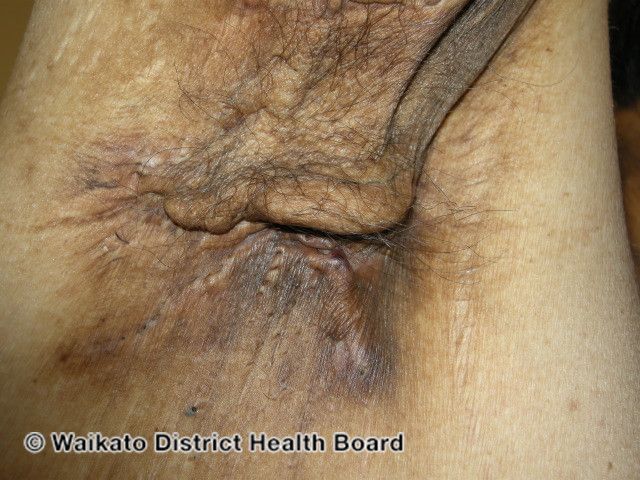
Sex Differences Contribute to Immunological Functioning Influences HS
Female sex bias in hidradenitis suppurativa can be attributed to several contributing immunological factors.
The female sex bias in hidradenitis suppurativa (HS) is probably multifaceted, with contributions from hormones, genetics, environmental influences, and the microbiome on changes in the immune system, according to a review1 in Frontiers in Immunology.
HS is a debilitating inflammatory disease that shows a strong female sex vias in multiple studies. It is characterized by immune dysregulation, including elevated levels of myeloid cells, T helper (Th) cells, and pro-inflammatory cytokines, especially those involved in Th1- and Th17-mediated immunity.
Despite this, former studies evaluating the immunological profiles of patients with HS have not noted stratified results between biologic females and males. Additionally, sex differences in the response to immunomodulatory HS biologics have not been extensively studied.
This review evaluated the role of intrinsic and extrinsic influences that add to immunological differences between the sexes and postulate their role in the female sex bias seen in HS. The researchers discuss the effects of hormones, X chromosome dosage, genetics, the microbiome, and smoking on sex-related differences in immunity to hypothesize possible immunological mechanisms in the pathophysiology of HS.
Of interest, the researchers noted that differences in sex-biased immune processes might explain some of the expanding literature supporting sex-biased comorbidities associated with HS. However, in some of the comorbidities, the sex difference was not significant, like psoriasis.
Further, although estrogens and androgens may have opposing roles in developmental processes, present data highlight their influence on the immune response is likely because of combined interactions and relative changes in concentrations leading to immune problems.
Growing numbers of studies involve a role for the microbiome in sex-biased immunity. Some studies have pinpointed sex-related alterations in the cutaneous microbiome, which may result from differences between the sexes in hormone metabolism, rates of perspiration, and skin pH. Additionally, differences in the immune system, like those previously described, might affect how females respond to changes in the microbiome.
Although antigen specificity has not been defined as a feature of HS, innate and humoral immune responses are obvious features of HS, and evidence of the effects of hormones, X-chromosome dosage, and sex-biased genes on immunological pathways emphasizes possible contributors to the female sex bias in HS. The clinical efficacy of cytokine blocking immunotherapy, like adalimumab, secukinumab, and brodalumab, supports a position for inflammatory cytokines as key disease mediators.
Hormonal fluctuations during puberty, the menstrual cycle, pregnancy, and menopause have been linked with the start of HS and/or HS flares in some patients, emphasizing that the effect of sex hormones on the cutaneous immune system may contribute to HS pathogenesis.
Multiple studies have also linked HS with acne, hirsutism, and polycystic ovarian syndrome, backing a role for androgens on disease. Additionally, recent case reports have explained new-onset and exacerbations of HS symptoms in gender dysphoric patients receiving supplemental testosterone therapy.
Of note, some patients report clinical improvement with anti-androgen therapy, but the specific roles of different hormones have not been well studied in HS pathophysiology. The researchers say that future studies elucidating the effects of sex hormones on inflammasome activation or immune cell function in patients with HS might support a mechanistic link for hormones in the mystery of HS pathophysiology.
More studies will assist in clarifying the molecular mechanisms of non-hormone and non-sex chromosome-related sex-biased gene expression.
Larger studies are needed to establish whether sex-related immunity linked with alterations in the gut microbiome might play a role in HS. Additionally, more studies are needed to explore the gut-skin axis and see if alterations in the gut microbiome may affect the cutaneous inflammation seen in HS.
The trend of male sex bias in HS was found from several studies in Asia, and probably represents a significant role of environmental influences on HS within the context of ancestry-specific genomic features. Smoking was described as one of the most substantiated environmental risk factors for HS.
In addition to the inflammation associated with smoking, chemicals like nicotine may disrupt skin metabolism, and future studies examining this might provide more context into how smoking might directly influence immune cells in patients with HS.
The researchers said that it will be vital to consider sex-based efficacy of immunomodulatory drugs in future trials in patients with HS considering the multiple potential sex-based immunological contribution to HS.
“A better understanding of sex-specific effects may also help reframe and improve management of HS in a more effective and equitable manner,” concluded the researchers.
Reference
- Young KZ, Dimitrion P, Zhou L, Adrianto I, Mi QS. Sex-biased immunological processes drive hidradenitis suppurativa. Front Immunol. Published online May 4, 2023. doi:10.3389/fimmu.2023.1167021
[This article was originally published by our sister publication, AJMC.]