- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
News
Article
Safety Profile of Secukinumab Favorable for Pediatric Patients With Plaque Psoriasis
Author(s):
In subgroups based on age and bodyweight, treatment with secukinumab did not create new safety signals.
To assess the safety of secukinumabin pediatric patients, researchers analyzed data from 2 phase 3 trials of patients with moderate to severe and severe plaque psoriasis and found secukinumab was well-tolerated.1 Authors pooled findings from 2 studies for a total of 198 pediatric patients and used findings from 4 adult studies for comparison of adverse events (AEs) between the age groups.
quayside/AdobeStock
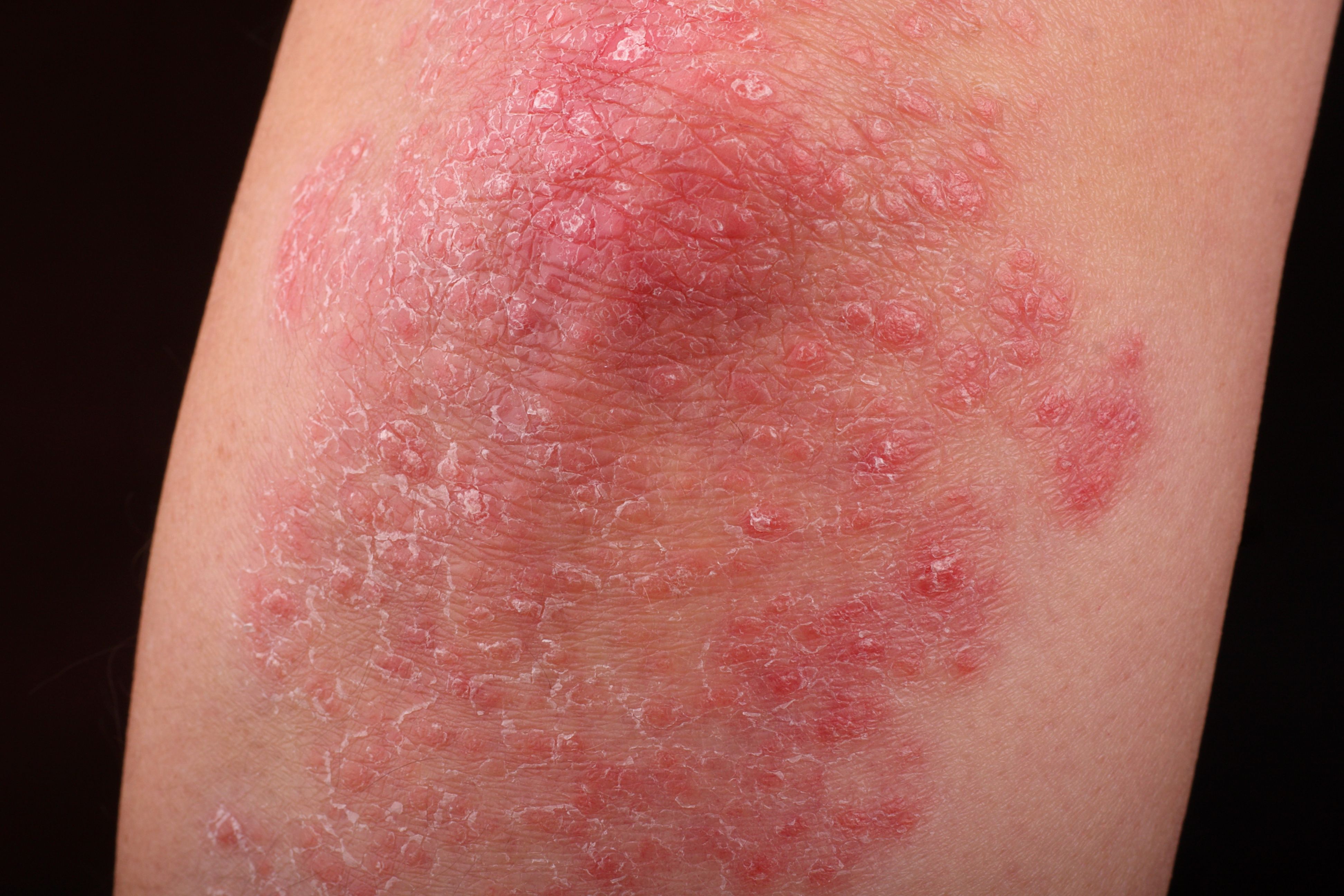
Pediatric patients were broken into subgroups by age, 6 to less than 12 and 12 to less than 18 years of age, and by bodyweight (<25 kg, 25 kg < 50 kg, and ≥ 50 kg). “Patients received secukinumab low dose (LD; 75/75/150 mg), secukinumab high dose (HD; 75/150/300 mg), placebo, or etanercept (0.8 mg/kg).”1 Severity of psoriasis was determined using the Psoriasis Area and Severity Index (PASI) and the Investigator’s Global Assessment (IGA).
Pediatric patients in both studies weighing less than 25 kg received 75 mg secukinumab in both LD and HD groups; patients weighing 25 to 50 kg received 75 mg (LD group) or 150 mg (HD group); and patients weighing ≥ 50 kg received 150 mg (LD group) or 300 mg (HD group).
Researchers broke down AEs into the categories of AEs, serious AEs, most frequent AEs by organ system class (SOC), most frequent AEs by preferred term (PT), AEs leading to treatment discontinuation, and AEs of special interest. Absolute neutrophil count and injection-site reactions (ISRs) were also assessed.
The secukinumab-treated pediatric group had the lowest exposure-adjusted incident rates (EAIRs) of treatment-emergent AEs (TEAEs) of any of the groups. “Up to 52 weeks, the IR [incidence rate] of AEs was 167.7/100 PY [patient years] in the secukinumab-treated patients in the < 12-year subgroup, which was lower than for the etanercept group (212.0/100 PY) and was also lower than the overall IR of AEs in the ≥ 12-year subgroup treated with secukinumab (214.7/100 PY).”1
The most common AE was nasopharyngitis. “Upper respiratory tract infections (URTIs) constituted a common PT AE and the most common HLT [High-Level Term] under the “Infections and infestations” SOC in the current analysis.”1 Candida infections were less common than in the adult population. None of the AEs resulted in discontinuation of the treatment. Researchers found that neutropenia was more common in pediatric groups than in adult groups.
AEs in the subgroups of age and bodyweight were similar to the general population, and no clinically significant differences were observed between subgroups. At 12 and 52 weeks, AEs were lower overall in the lower age and lower bodyweight subgroups.
A limitation of the studies was the small number of patients in some of the subgroups.
Authors concluded, “The results from this analysis further demonstrate the favorable safety profile of secukinumab in pediatric patients with moderate to severe and severe chronic plaque psoriasis.”1
Reference
- Sticherling M, Nikkels AF, Hamza AM, et al. Secukinumab in pediatric patients with plaque psoriasis: Pooled safety analysis from two phase 3 randomized clinical trials. Am J Clin Dermatol (2023). https://doi.org/10.1007/s40257-023-00782-8
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.







