- General Dermatology
- Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
Remote Assessments Show Crisaborole Ointment Eases Stasis Dermatitis
Patients treated with the ointment showed improvement in lesional percentage body surface area.
Andrew/AdobeStock
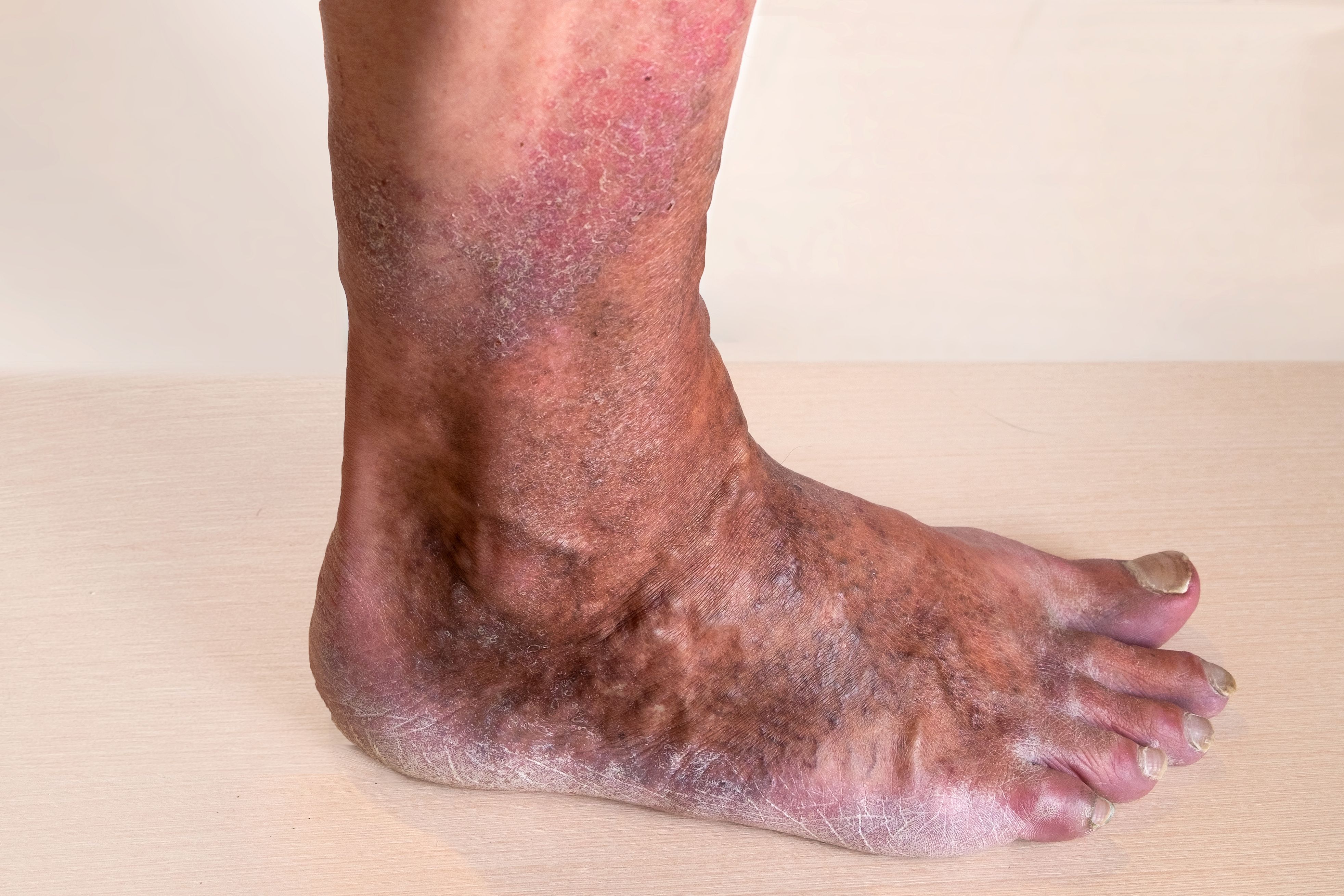
In a recent study of 65 patients with stasis dermatitis (SD, also known as venous eczema), those who were treated with crisaborole ointment, 2% (Eucrisa) showed improvement when assessed by dermatologists examining digital images.1
The double-blind, vehicle-controlled, parallel-group, proof-of-concept phase 2a study (NCT04091087) included participants 45 years old or older with stasis dermatitis but without active ulceration. All participants had to have active unilateral or bilateral stasis dermatitis lesions with involved areas totaling ≥1% of body surface area (BSA) at screening. Participants were randomly assigned to receive crisaborole ointment, 2%or vehicle (1:1) in an even layer of ointment (∼3 mg/cm2) twice daily for 6 weeks.
The researchers were led by Jonathan I. Silverberg MD, PhD, MPH, an associate professor in the department of dermatology at the George Washington University School of Medicine and Health Sciences in Washington, DC. The study was posted online February 8 in the Journal of the American Academy of Dermatology.
Crisaborole ointment, 2%, is a nonsteroidal topical phosphodiesterase 4 inhibitor approved for the treatment of mild to moderate atopic dermatitis.
SD tends to develop in older patients due to chronic venous insufficiency and hypertension. Although prompt SD treatment may prevent serious skin complications, including venous ulcers, Silverberg et al noted, no treatments are specifically approved for SD treatment, so a need exists for novel and effective therapies with favorable safety profiles.
Study Method
Patient encounters included 3 in-home visits and 6 telemedicine interactions. The crisaborole ointment, 2% was shipped to the participants' homes, and high-resolution digital photography was used to allow “central readers” (board-certified dermatologists with expertise in venous disease and SD) to remotely assess SD lesion severity.
In-person safety and efficacy assessments were performed by home visit practitioners (HVPs). These HVPs were medically qualified nondermatologists with training and clinical experience in the identification of SD. They had completed training modules demonstrating the use of the efficacy rating scales and completed rater training and standardization exercises.
Results
Silverberg et al reported, “Efficacy according to success and improvement per Investigator's Global Assessment score and lesional percentage body surface area reached statistical significance based on central reader but not in-person assessments.”
Specifically, patients treated with crisaborole had a larger percentage change from baseline in lesional %BSA at week 6 versus vehicle as assessed by dermatologists (−34.3% vs 18.7%; difference: −53.01%; 90% CI [−103.26, −2.75]; P = .0416) than as assessed by nondermatologists (−3.8% vs −10.7%; difference: 6.91%; 90% CI [−9.23, 23.06]; P = .2383).
Crisaborole was found to have an acceptable tolerability profile, with most adverse events (AEs) being of mild to moderate severity, the researchers reported. "Crisaborole offers a promising well-tolerated therapeutic approach to alleviate the skin complications associated with SD,” they wrote.
Skin and subcutaneous tissue disorders, namely pruritus (n = 3; 9.1%), erythema (n = 2; 6.1%), and contact dermatitis (n = 2; 6.1%), were the most common all-causality treatment-emergent adverse events in those treated with crisaborole. Serious and severe AEs were each experienced by 1 participant (3%) treated with crisaborole and 4 participants (12.5%) treated with vehicle. No participants discontinued the study due to AEs.
“Although the [nondermatologists] had experience with SD and received training,” the researchers commented, “it is possible that differences in expertise between [nondermatologists] and [dermatologists] may have contributed to the limited agreement between in-person and remote assessments.
“Nevertheless, these data suggest fully decentralized, siteless clinical trials can be successfully implemented. Decentralized clinical trials facilitate the recruitment and retention of participants by removing geographic and travel-related barriers that may preclude in-person site visits.2 Remote monitoring through digital technologies may reduce operational costs and offer investigators greater procedural simplicity and flexibility.”
The study was funded by Pfizer.
References
- Silverberg JI, Kirsner RS, David J. Margolis DJ, et al. Efficacy and safety of crisaborole ointment, 2%, in participants aged ≥45 years with stasis dermatitis: Results from a fully decentralized, randomized, proof-of-concept phase 2a study. Journal of the American Academy of Dermatology. 2024. ISSN 0190-9622.https://doi.org/10.1016/j.jaad.2023.12.048.
- Ali Z, Zibert JR, Thomsen SF. Virtual clinical trials: perspectives in dermatology. Dermatology. 9 July 2020; 236 (4): 375–382. https://doi.org/10.1159/000506418
