- General Dermatology
- Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
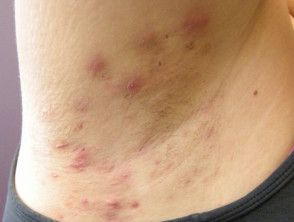
Lutikizumab Demonstrates Positive Results in Phase 2 Trial for Hidradenitis Suppurativa
Higher response rates were observed in patients treated with lutikizumab. The drug will now advance to phase 3 clinical trials.
AbbVie announced today positive data stemming from its phase 2 clinical trial of lutikizumab (ABT-981) in adult patients with moderate to severe hidradenitis suppurativa (HS). AbbVie also announced that in response, its clinical program of lutikizumab for HS will advance to phase 3.
The investigational, dual-variable-domain interleukin 1α/1β antagonist achieved higher response rates in patients who had previously failed anti-TNF therapy versus a placebo.
The 16-week randomized, double-blinded, multicenter, placebo-controlled, dose-ranging, and parallel-group trial enrolled 153 adult patients with moderate to severe HS. At baseline, approximately 70.6% of participants had HS characterized by a Hurley staging of 3, indicative of extensive scarring, lesions, and sinus tracts.
Patients were randomized to 1 of 4 treatment groups: 100 mg lutikizumab every other week, 300 mg every other week, 300 mg every week, and a placebo.
At the conclusion of the 16 weeks, all doses of lutikizumab had achieved higher response rates regarding the trial's primary endpoint of HiSCR 50.
While 100 mg lutikizumab dosed every other week did not demonstrate greater efficacy compared to the placebo, both 300 mg doses (every week and every other week) exhibited higher rates of improved skin pain via NRS30 and HiSCR75 scoring, as well as a higher threshold of HS clinical response.
"The burdens of HS are high and include long times to diagnosis, significant pain, disability, isolation, and reduced quality of life," said Alexa B. Kimball, MD, MPH, a study investigator from Beth Israel Deaconess Medical Center in Boston and Professor of Dermatology at Harvard Medical School. "These results are encouraging and help us further understand the use of lutikizumab in patients with HS as we work to address the need for additional treatment options for patients living with this disease."
All doses of lutikizumab were generally well-tolerated, with similar rates of treatment-emergent adverse events (TEAEs) reported among combined lutikizumab treatment arms. The most common TEAEs reported were HS, diarrhea, headache, pruritus, contact dermatitis, eczema, and nasopharyngitis among patients dosed with lutikizumab.
Serious adverse events were reported in approximately 5.3% of patients receiving lutikizumab.
"AbbVie continues to pioneer research in the pursuit of new treatment options for patients with hidradenitis suppurativa, a frequently overlooked, underserved, and often suffering patient population," said Roopal Thakkar, MD, senior vice president, chief medical officer, global therapeutics, at AbbVie, in a press release. "These results help us further understand the use of lutikizumab in adults with moderate to severe hidradenitis suppurativa, and we will continue to apply our more than 25 years of expertise in immune-mediated diseases in advancing our clinical program for lutikizumab in HS to Phase 3."
Reference
AbbVie. Lutikizumab showed positive results in a phase 2 trial of adults with moderate to severe hidradenitis suppurativa as program advances to phase 3. PR Newswire. January 8, 2024. Accessed January 8, 2024. https://www.prnewswire.com/news-releases/lutikizumab-showed-positive-results-in-a-phase-2-trial-of-adults-with-moderate-to-severe-hidradenitis-suppurativa-as-program-advances-to-phase-3-302027605.html