- General Dermatology
- Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
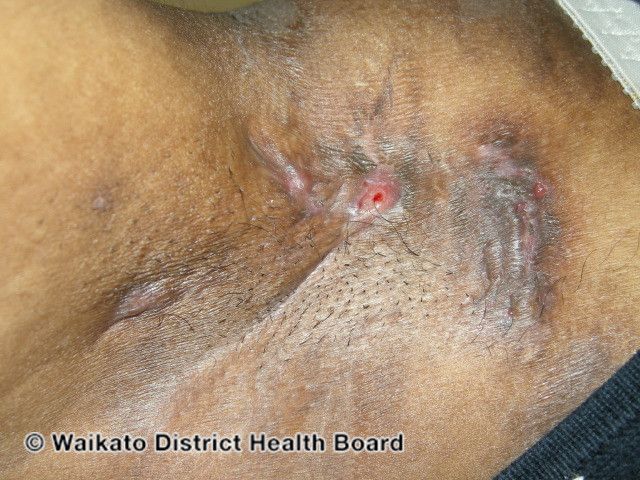
Investigating Intravenous Ertapenem Therapy for Hidradenitis Suppurativa
IV antibiotics should play a complementary role in the management of HS as new immunomodulator therapies are developed, researchers concluded.
A new study looking into the use of IV ertapenem for recalcitrant hidradenitis suppurativa (HS) found that administration of the antibiotic for an average of 13 weeks resulted in improvements in clinical and inflammatory markers, as well as improved patient satisfaction.1
Avigdor Nosrati, MD, et al in 2019 reported short-lived efficacy after 6 weeks of treatment with ertapenem; this duration is the current standard of care recommended in the North American treatment guidelines for HS.2,3
Findings regarding recurrent disease activity and pain severity within 4 weeks of discontinuing ertapenem led the researchers to explore the durability of HS remissions after longer treatment intervals, they reported.
To perform the new research, Nosrati et al conducted a retrospective review of the medical records of 98 patients with HS treated at the Albert Einstein College of Medicine’s Montefiore HS Center in New York between 2018 and 2022.
Patient outcomes before and after treatment with IV ertapenem were measured, and participants were followed up in a phone survey.
The patients’ mean age was 35.8 years, and 61 (62.2%) were female.
The self-reported racial distribution included:
- 59 patients who identified as Black/African American (60.2%)
- 24 who identified as Spanish/ Hispanic/Latino (24.5%)
- 23 “other” or “unknown” (23.5%)
- 13 who identified as White (13.3%)
- 3 who identified as Asian (3.1%)
Study Rationale and Methods
The Montefiore HS Center treatment algorithm consists of 3 core therapies: antibiotics (topical and/or oral), antiandrogen therapies (spironolactone, finasteride, and/or oral contraceptive pills), and biologics (adalimumab and infliximab), Nosrati et al reported.
“Although oral antibiotics are generally accepted as a core therapeutic approach to HS, much less is known about the efficacy of IV antibiotics, especially ertapenem, a parenteral carbapenem possessing activity against many gram-positive bacteria, gram-negative bacteria, and anaerobic organisms,” they wrote.
Overall, 78 participants (80%) in this study were taking 1 or more medications at baseline, and 74 (76%) continued this combination (excluding oral antibiotics) during the ertapenem treatment course.
At the initial visit, the range of treatments the patients were receiving included topical antibiotics (n = 97); oral antibiotics (n = 75); spironolactone (n = 44); finasteride (n = 41); oral contraceptive pills (n = 25); adalimumab (n = 9); and infliximab (n = 78), Nosrati et al reported.
These therapies were maintained during the course of ertapenem as follows: topical antibiotics (n = 95; 98%); spironolactone (n = 39; 89%); finasteride (n = 22; 90%); oral contraceptive pills (n = 22; 88%); adalimumab (n = 8; 89%); and infliximab (n = 75; 96%).
Laboratory markers of inflammation were recorded at baseline, midcourse, end-of-course, and posttherapy follow-up, and swab bacterial culture results from actively draining sites were recorded at each visit.
Study Results
Adverse events associated with IV ertapenem therapy were uncommon. Of the 98 patients, 8 (8.2%) had diarrhea, 5 (5.1%) had nausea, 2 (2%) had headaches, 1 (1%) had candidiasis, and 1 (1%) had multiple episodes of syncope.
Of the patients, 76 (77.6%) participated in a phone survey; of these, 63 (80.3%) were satisfied or very satisfied with ertapenem therapy. When respondents were asked if they would be willing to receive another course of therapy, 60 (78.9%) responded affirmatively; furthermore, 69 (90.8%) would recommend ertapenem to other patients, Nosrati et al reported.
The findings suggest a course of 12 to 16 weeks of ertapenem may be appropriate as a new standard length of therapy in HS patients, which is at least twice the current recommendation of North American treatment guidelines, they concluded.
Nosrati told Dermatology Times, “Our research contributes to the existing literature and, more crucially, to the well-being of our HS patients, many of whom endure profound suffering. Our study provides valuable insights into both the short- and longer-term clinical outcomes and subjective experiences of patients undergoing intensive ertapenem treatment.”
Addressing Antibiotic Resistance
Regarding the problem of antimicrobial resistance, Nosrati said, “In treating our most recalcitrant and severe cases of HS, we observed a remarkable improvement in quality of life during and after ertapenem therapy. The impact of HS extends well beyond its visible manifestations, leading to a range of primary and secondary complications such as opioid use disorder, psychological issues, and aggressive squamous cell carcinomas.
“Consequently, the advantages and risks of employing ertapenem need careful consideration. In our study, the occurrence of antibiotic resistance was minimal; however, the emergence of carbapenemase-producing organisms, such as Pseudomonas and Enterococcus species, led us to later suppress these secondary invaders with adjunctive oral antibiotics, including linezolid.
“Nonetheless, the high degree of efficacy and patient satisfaction reflect the substantial short- and long-term enhancements in quality of life and overall disease burden, providing a compelling rationale for the use of ertapenem.”
References
- Nosrati A, Ch’en PY, Torpey ME, et al. Efficacy and durability of intravenous ertapenem therapy for recalcitrant hidradenitis suppurativa. JAMA Dermatol. Published online February 14, 2024. doi:10.1001/jamadermatol.2023.6201
- Nosrati A, Babbush KM, Ghias MH, et al. Short-lived efficacy of ertapenem for refractory hidradenitis suppurativa. Presented at: Fourth Annual Symposium on Hidradenitis Suppurativa Advances; November 2019; Detroit, MI.
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81(1):91-101. doi:10.1016/ j.jaad.2019.02.068